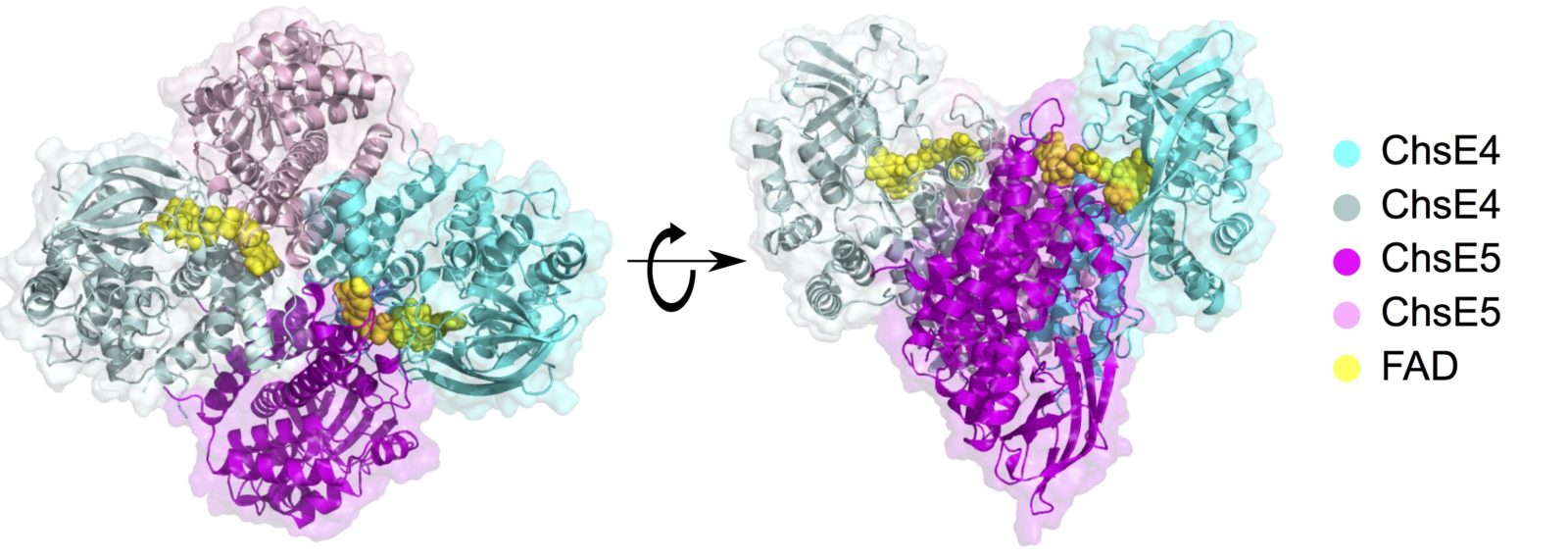
image from: Yang, M., Lu, R. Guja, K. E., Wipperman, M., St. Clair, J. Bonds, A., Garcia-Diaz, M. And Sampson, N. S. (2015) “Unraveling cholesterol catabolism in Mycobacterium tuberculosis: the ChsE4-ChsE5 α2β2 acyl-CoA dehydrogenase initiates β-oxidation of 3-oxo-cholest-4-en-26-oyl CoA,” ACS Infectious Disease, 1, 110-125. DOI: 10.1021/id500033m
Cholesterol metabolism is one of the mechanisms by which mycobacteria, e.g., Mycobacterium tuberculosis (Mtb), survive and persist in their human host. Our laboratory initiated an investigation of the entire cholesterol metabolism pathway in Mtb using a multi-pronged approach that includes elucidating enzyme function, establishing metabolite structure and activity to identify the substrates of key enzymes, understanding pathway regulation, and identifying small molecules that interfere with the survival of Mtb in the human host. We identified unique structural assemblies of two enzyme classes in Mtb.