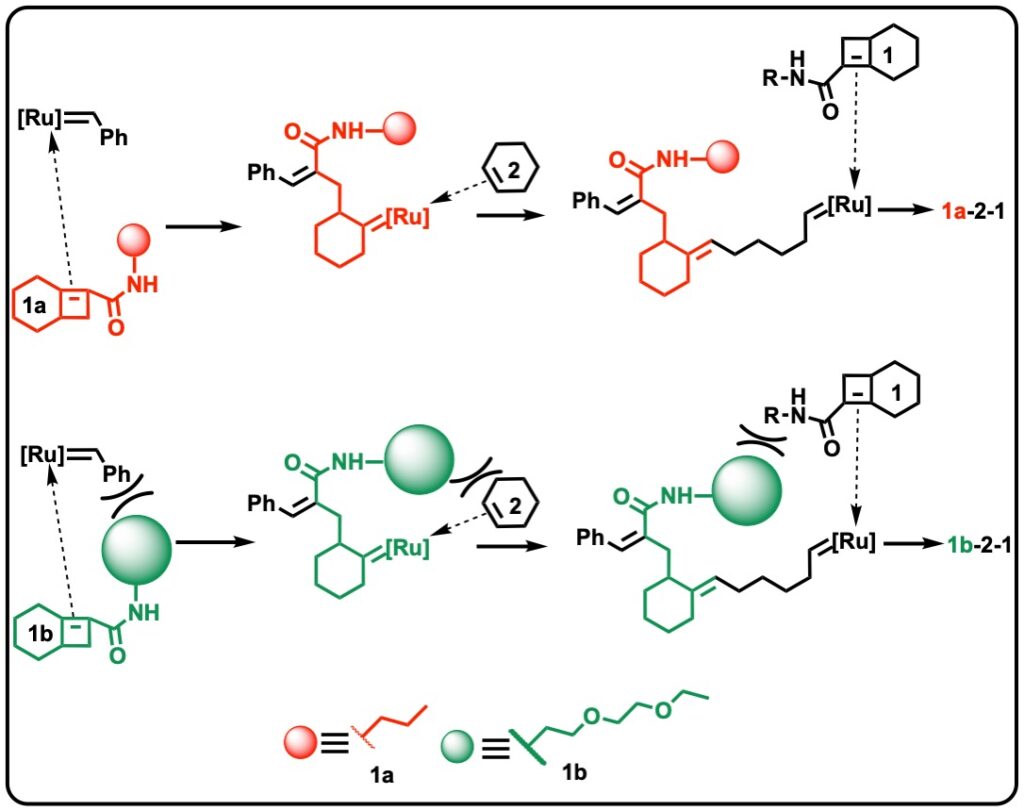
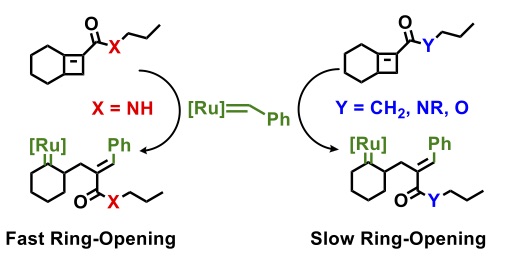
Boadi, F., and Sampson, N. S. (2023) “Long-range kinetic effects on the alternating ring opening metathesis of bicyclo[4.2.0]oct-6-ene-7-carboxamides and cyclohexene,” in press ACS Organic and Inorganic Au DOI: 10.1021/acsorginorgau.3c00013 PMCID in progress
Yu, X., Li, G., Zheng, B., Youn, G., Jiang, T., Quah, SP., Laughlin, S.T., Sampson, N. S. and Bhatia, S. R. (2022) “Controlling rheology of fluid interfaces through microblock length of sequence-controlled amphiphilic copolymers,” 223, 2200110, Macromolecular Chemistry and Physics. DOI: 10.1002/macp.202200110 PMC9799073.
Yang, X, Wipperman, M. F., Nachman S., Sampson, N. S. (2022) “Exploring the value of Mycobacterium tuberculosis modified lipoprotein as a potential biomarker for TB detection in children,” BMC Infectious Diseases, 22, article # 158. DOI: 10.1186/s12879-022-07140-9, https://rdcu.be/cHfoR PMC8851740
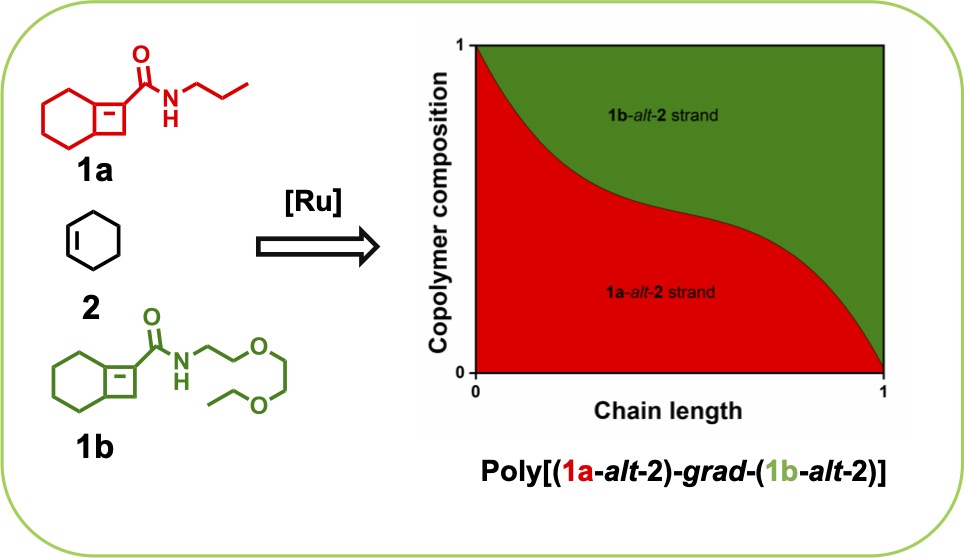
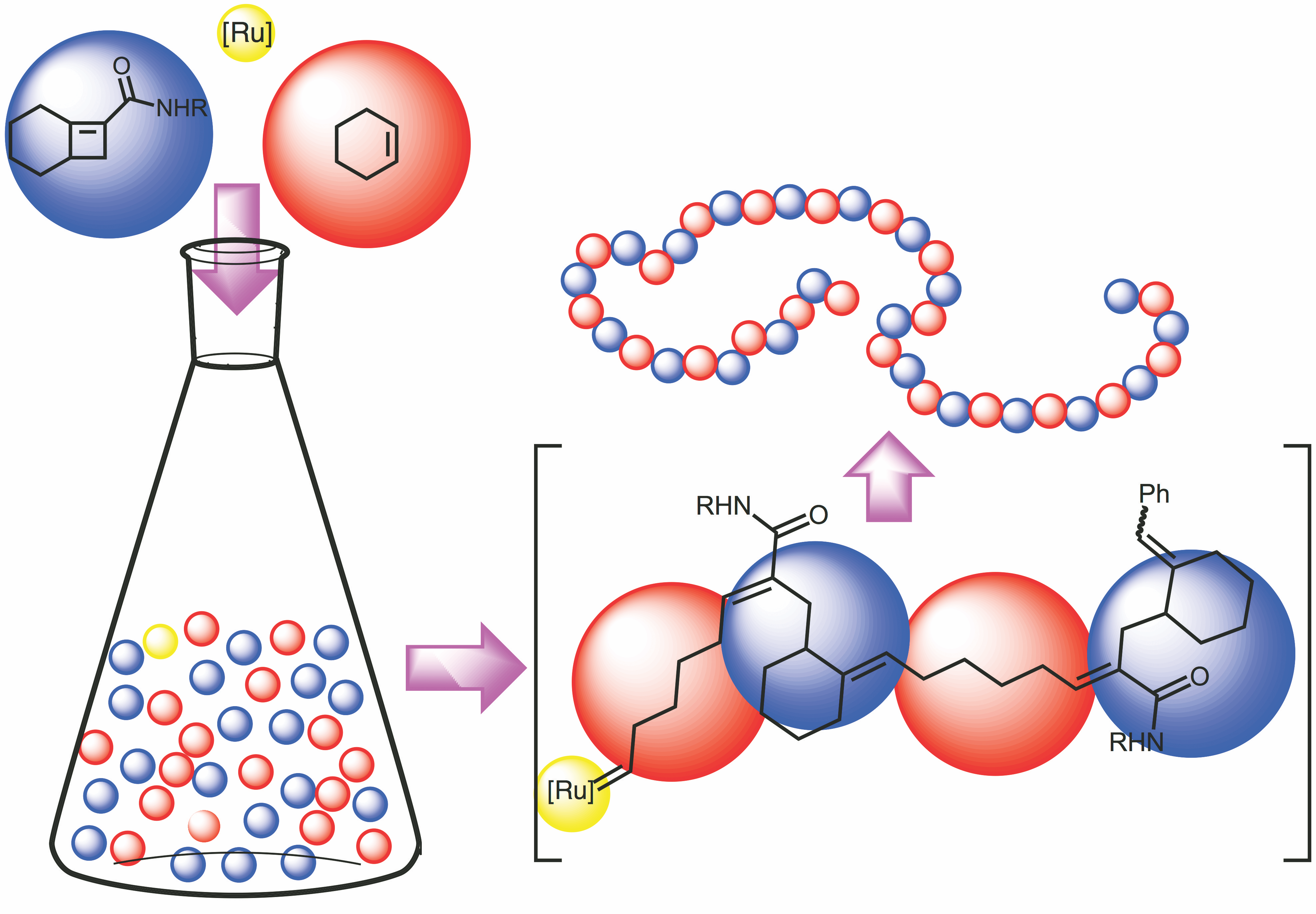
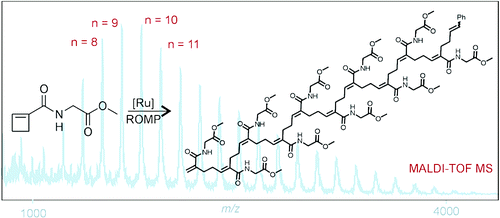
Boadi, F. O., Sampson, N. S. (2021) “Gradient copolymer prepared from alternating ring-opening metathesis of three monomers” 12, 5613 – 5622, Polymer Chemistry DOI:10.1039/D1PY00690H
Youn, G., Sampson, N. S. (2021) “Substituent effects provide access to tetrasubstituted ring-opening olefin metathesis of bicyclo[4.2.0]oct-6-enes,” 1, 29-36, ACS Organic and Inorganic Au. DOI: 10.1021/acsorginorgau.1c00016.
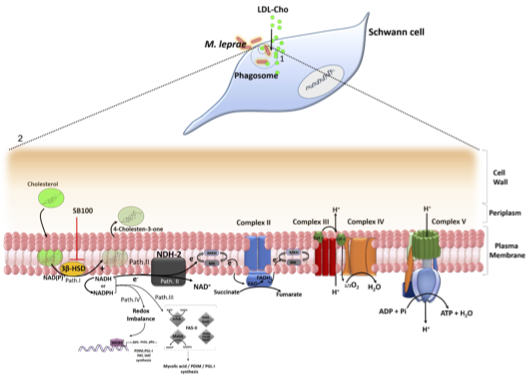
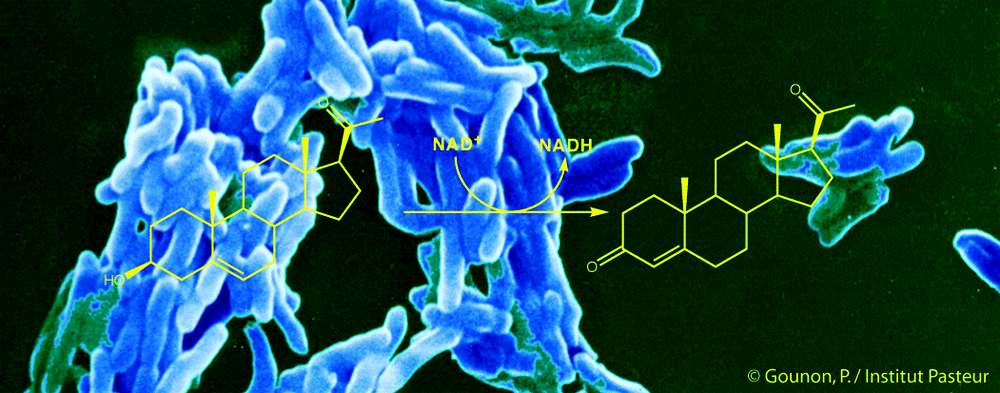
Rosa, T.L.S.A., Marques, M.A.M., DeBoard, Z., Hutchins, K.M., Silva, C.A.M., Montague, C.R., Yuan, T., Amaral, J.J., Atella, G. C., Rosa, P. S., Mattos, K. A., VanderVen, B. C., Lahiri, R., Sampson, N. S., Brennan, P., Belisle, J. T., Pessolani M. C. V., and Berredo-Pinho, M* (2021) “Reductive power generated by Mycobacterium leprae through cholesterol oxidation contributes to lipid and ATP synthesis,” Front. Cell. Infect. Microbiol. – Bacteria and Host. 11, 701, DOI: 10.3389/fcimb.2021.709972.
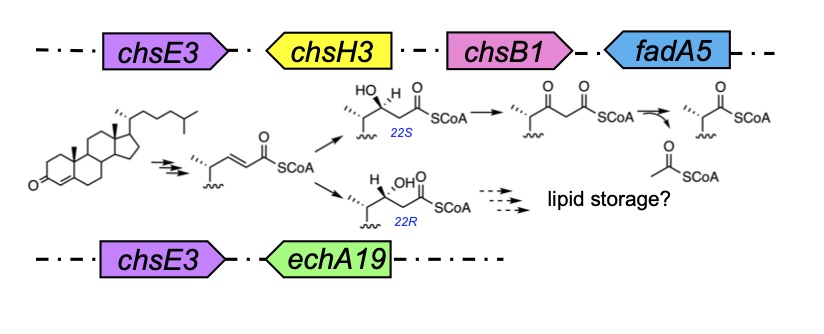
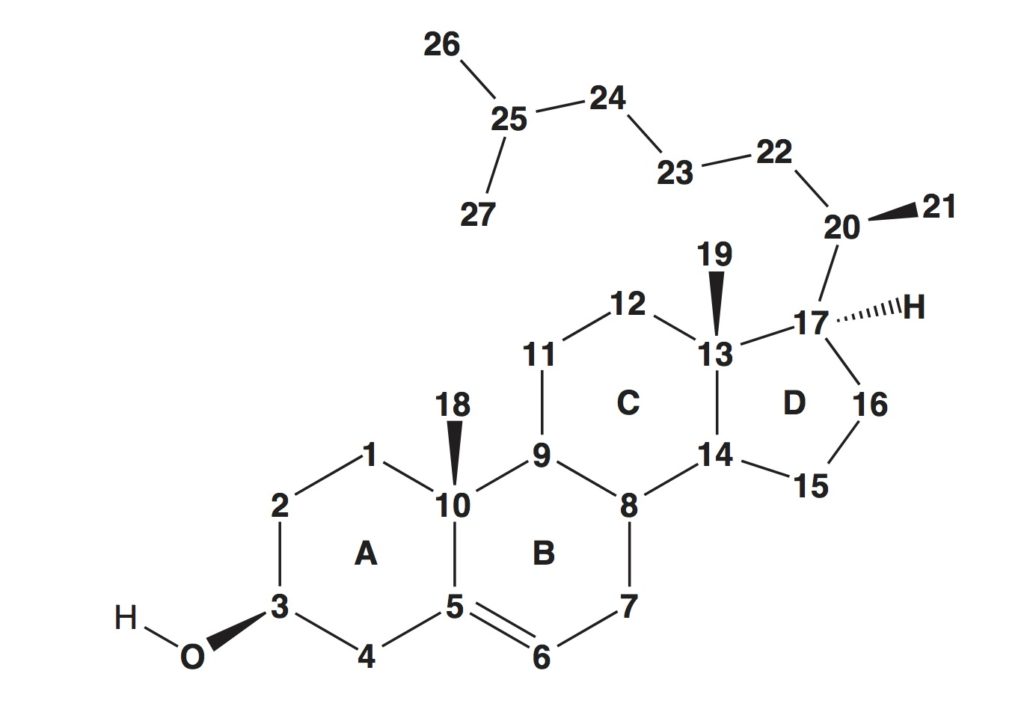
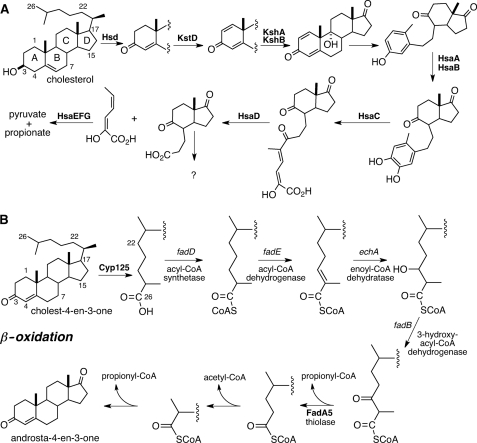
Yuan , Τ., Werman, J. M., Yin, X., Yang, M., Garcia-Diaz, M., Sampson, N. S. (2021) “Enzymatic β-oxidation of the cholesterol side chain in Mycobacterium tuberculosis bifurcates stereospecifically at hydration of 3-oxo-cholest-4,22-dien-24-oyl-CoA,” ACS Infectious Diseases. 7, 1739-1751. DOI: 10.1021/acsinfecdis.1c00069.
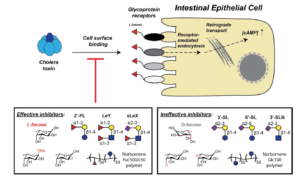
Youn, G., Cervin, J., Yu, X., Bhatia,S. R., Yrlid, U. and Sampson, N. S., (2020) “Targeting multiple binding sites on cholera toxin B with glycomimetic polymers promotes formation of protein–polymer aggregates,” Biomacromolecules 21, 4878-4887. DOI: 10.1021/acs.biomac.0c01122
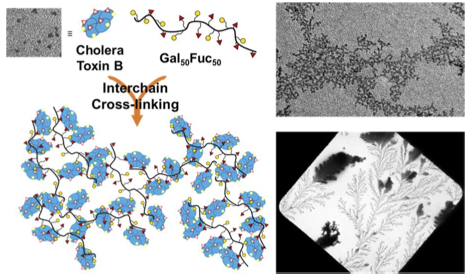
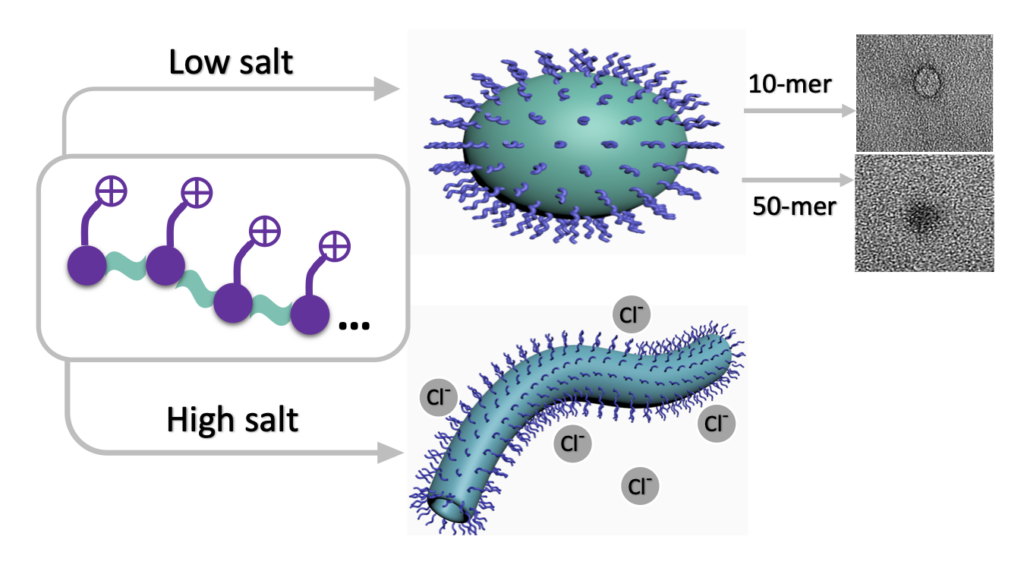
Zhang, J., Yu, X., Zheng, B., Shen, J., Bhatia, S. R., and Sampson, N. S. (2020) “Cationic amphiphilic alternating copolymers with tunable morphology,” Polymer Chemistry 11, 5424-5430. DOI: 10.1039/D0PY00782J
Cervin, J., Boucher, A., Youn, G., Björklund, P., Wallenius, V., Mottram, L., Sampson, N. S., and Yrlid, U. (2020) “Fucose-galactose polymers inhibit cholera toxin binding to fucosylated structures and galactose-dependent intoxication of human enteroids, ACS Infectious Diseases 6, 1192-1203, DOI: 10.1021/acsinfecdis.0c00009
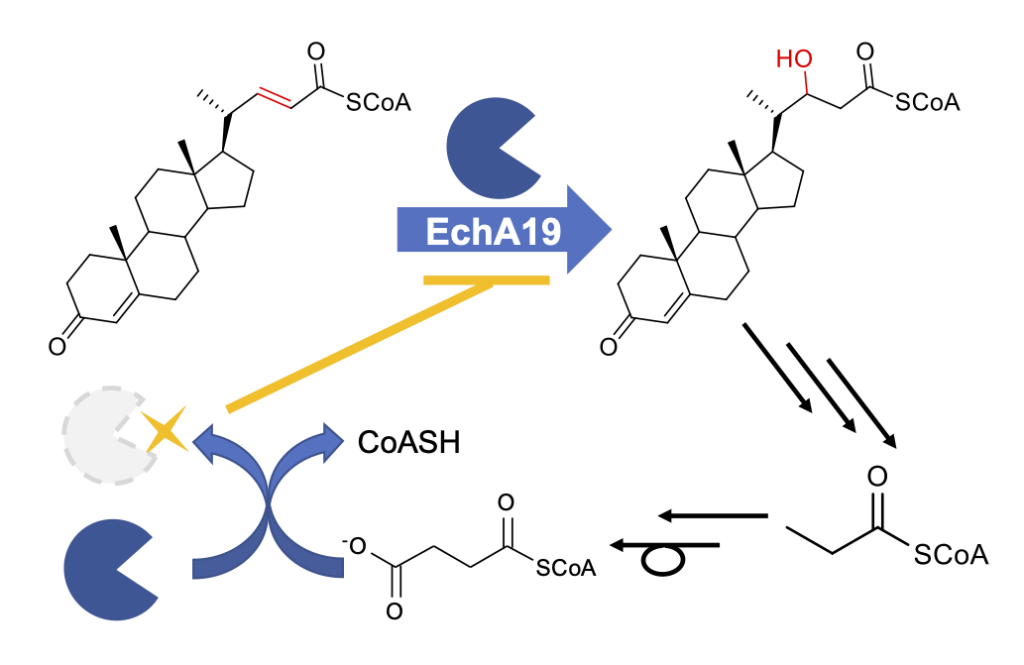
Bonds, A, Yuan, T. Werman, J. M., Jung, J, Lu, R., Nesbitt, N. M., Werman, J., Garcia-Diaz, M., and Sampson, N. S. (2020) “Post-translational succinylation of Mycobacterium tuberculosis enoyl-CoA hydratase EchA19 slows catalytic hydration of cholesterol catabolite 3-oxo-chol-4,22-diene-24-oyl-CoA,” ACS Infectious Diseases. 6, 2214-2224, DOI: 10.1021/acsinfecdis.0c00329
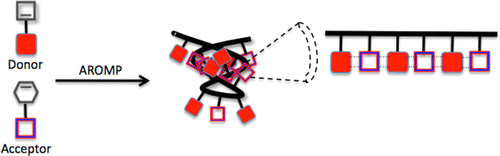
Boadi, F. O., Zhang, J., Yu,X., Bhatia,S. R., and Sampson, N. S. (2020) “Alternating ring-opening metathesis polymerization provides easy access to functional and fully degradable polymers,” Macromolecules. 53, 5857-5868, DOI: 10.1021/acs.macromol.0c01051
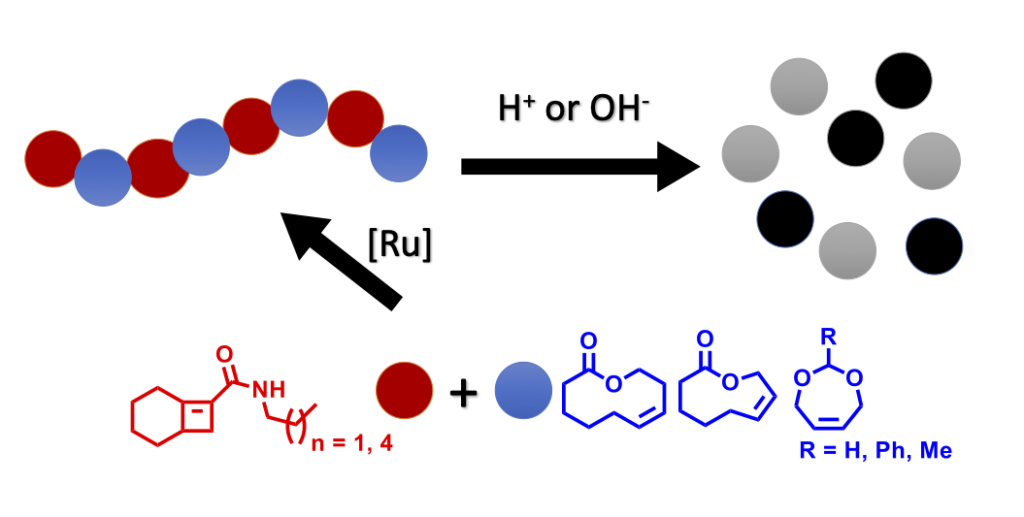
Gadbery, J. E., Round, J. W., Wipperman, M. F., Yuan, T., Story, K. T., Crowe, A. M., Casabon, I., Liu, J., Yang, X., Eltis, L. D., and Sampson, N. S. (2020) “IpdE1-IpdE2 is a heterotetrameric acyl coenzyme A dehydrogenase that is widely distributed in steroid-degrading bacteria”, Biochemistry. 59, 1113-1123 DOI: 10.1021/acs.biochem.0c00005
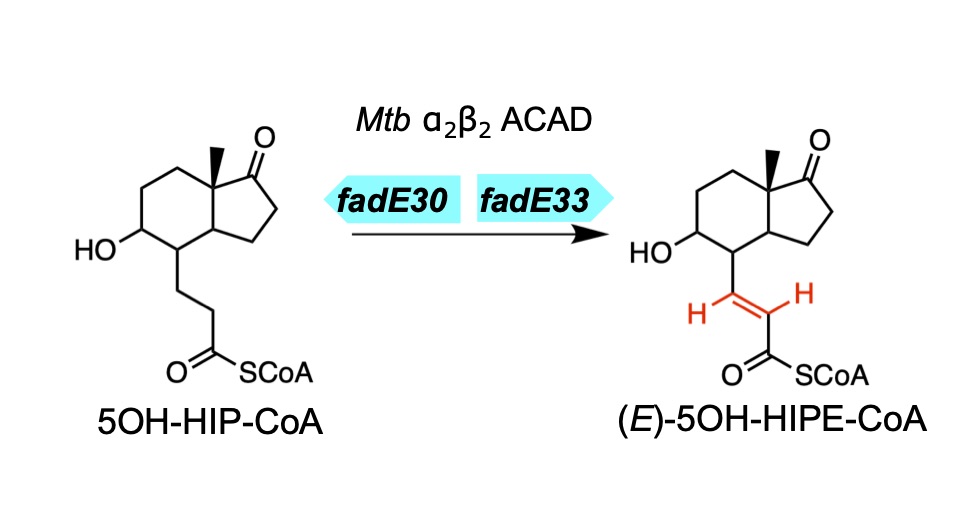
Yuan, T., Yang, M., Gehring, K., and Sampson, N. S. (2019) “Mycobacterium tuberculosis exploits a heterohexameric enoyl-CoA hydratase retro-aldolase complex for cholesterol catabolism,” Biochemistry. DOI: 10.1021/acs.biochem.9b00673
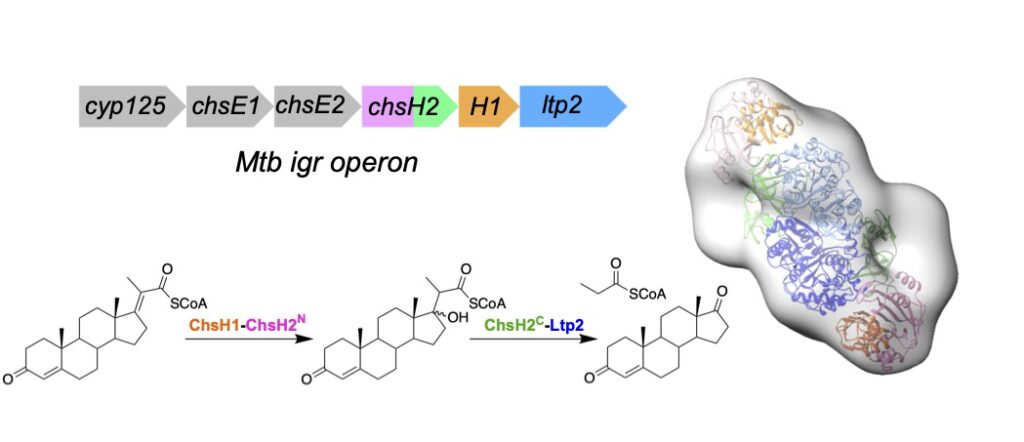
Gadbery, J. and Sampson, N. S. (2019) “Applications of ICMT for protein structure determination,” Meth. Enzymol. 621, 245-260. DOI: 10.1016/bs.mie.2019.02.02
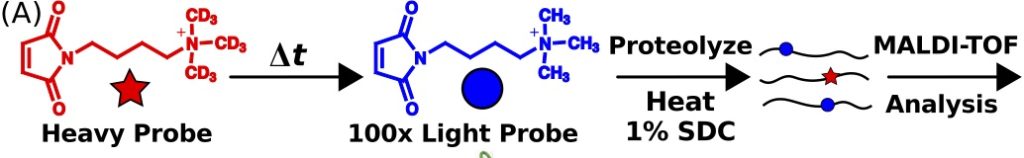
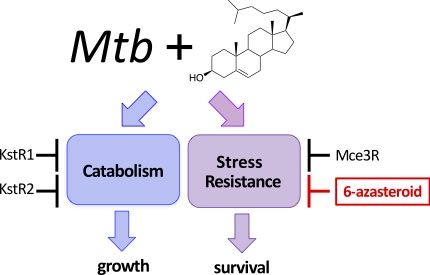
Yang, X, Yuan, T., Ma, R., Chacko, K. I., Smith,M., Deikus,G. Sebra, R., Kasarskis, A., van Bakel, H., Franzblau, S., and Sampson, N. S. (2019) “The Mce3R stress-resistance pathway is vulnerable to small-molecule targeting that improves tuberculosis drug activities,” in press ACS Infect. Dis. 10.1021/acsinfecdis.9b00099
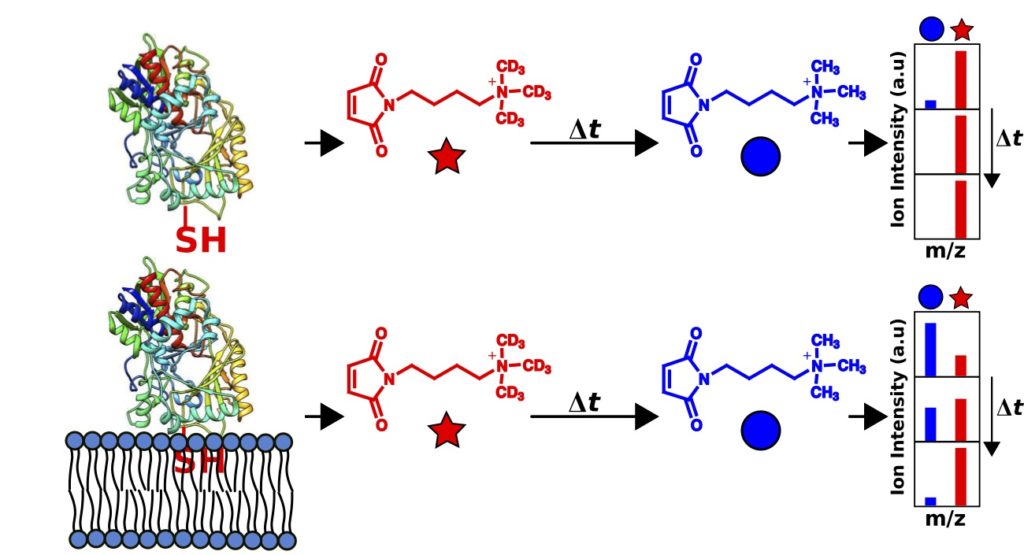
Gadbery, J, and Sampson, N. S. (2018) “Use of an Isotope-Coded Mass Tag (ICMT) Method to Determine the Orientation of Cholesterol Oxidase on Model Membranes,“ 57, 5370-5378, Biochemistry.DOI: 10.1021/acs.biochem.8b00788 PMC6171977
Zhang, J., Li, G., and Sampson, N. S. (2018) “Incorporation of large cycloalkene rings into alternating copolymers allows control of glass transition and hydrophobicity,” 7,1068–1072,ACS Macro. Lett. DOI: 10.1021/acsmacrolett.8b00510 PMC6156091
Li, G. and Sampson, N. S. (2018) “Alternating Ring-Opening Metathesis Polymerization (AROMP) of Hydrophobic and Hydrophilic Monomers Provides Oligomers with Side-Chain Sequence Control,” 51, 3932–3940, Macromolecules. DOI: 10.1021/acs.macromol.8b00562. PMC6262599
Bonds, A. and Sampson, N. S. (2018) “More than catabolism: Regulatory vulnerabilities in Mycobacterium tuberculosis,”Curr. Opin. Chem. Biol. 44, 39-46. DOI: 10.1016/j.cbpa.2018.05.012
Wands, A.M., Cervin, J., Huang, H., Zhang, Y., Youn, G., Brautigam, C. A., Dzebo, M. M., Bjorklund, P. Wallenius, V., Bright, D.K., Bennett, C.S., Wittung-Stafshede, P., Sampson, N.S., Yrlid, U., and Kohler, J.J. (2018) “Fucosylated molecules competitively interfere with cholera toxin binding to and intoxication of host cells,” 4, 758-770, PMC5948155, ACS Infect. Disease. DOI: 10.1021/acsinfecdis.7b00085
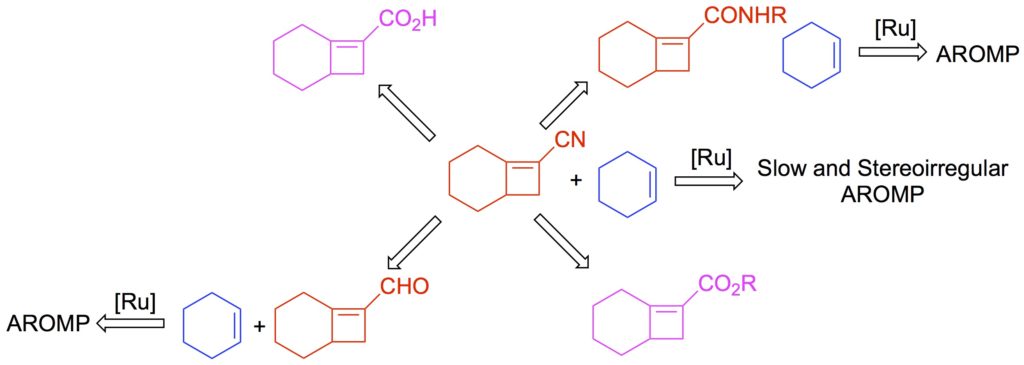
Chen, L., Liu, L., and Sampson, N. S. (2018) “Access to bicyclo[4.2.0]octene monomers to explore the scope of alternating ring-opening metathesis polymerization,” 83 2892–289. J. Org. Chem. DOI: 10.1021/acs.joc.8b00054.
monomers may be purchased through Kerafast:
https://www.kerafast.com/
https://www.kerafast.com/
,1887-1916. DOI: 10.1021/ acs.chemrev.7b00602
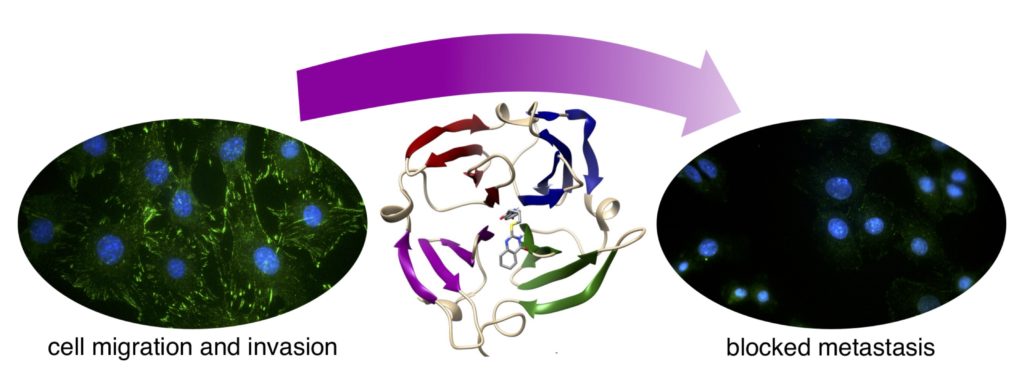
Alford, V. M., Kamath, A., Ren, X. Kumar, K., Gan, Q., Awwa, M., Tong, M., Seeliger, M., Cao, J., Ojima, I., and Sampson, N.S. (2017) “Targeting the hemopexin-like domain of latent matrix metalloproteinase-9 (proMMP-9) with a small molecule inhibitor prevents the formation of focal adhesion junctions,” 12, 2788-2803, ACS Chemical Biology DOI: 10.1021/acschembio.7b00758
Rui Lu, Christin M. Schaefer, Natasha M. Nesbitt, Jochen Kuper, Caroline Kisker, and Nicole S. Sampson
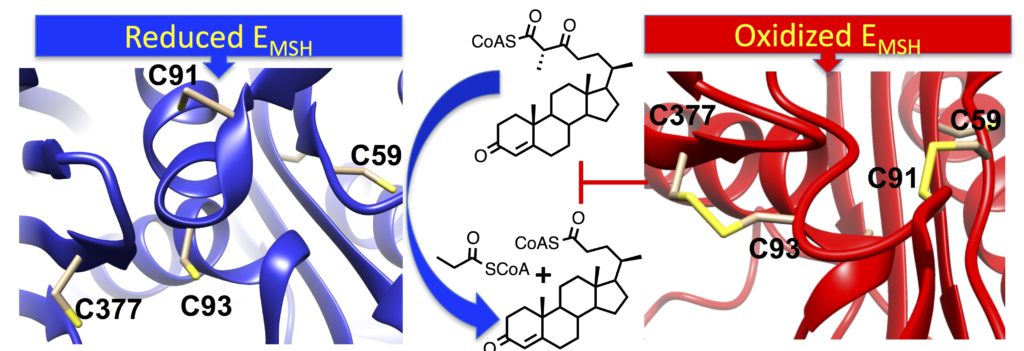
“Catabolism of the Cholesterol Side Chain in Mycobacterium tuberculosis is Controlled by a Redox-Sensitive Thiol Switch,” ACS Infectious Diseases, DOI: 10.1021/acsinfecdis.7b00072
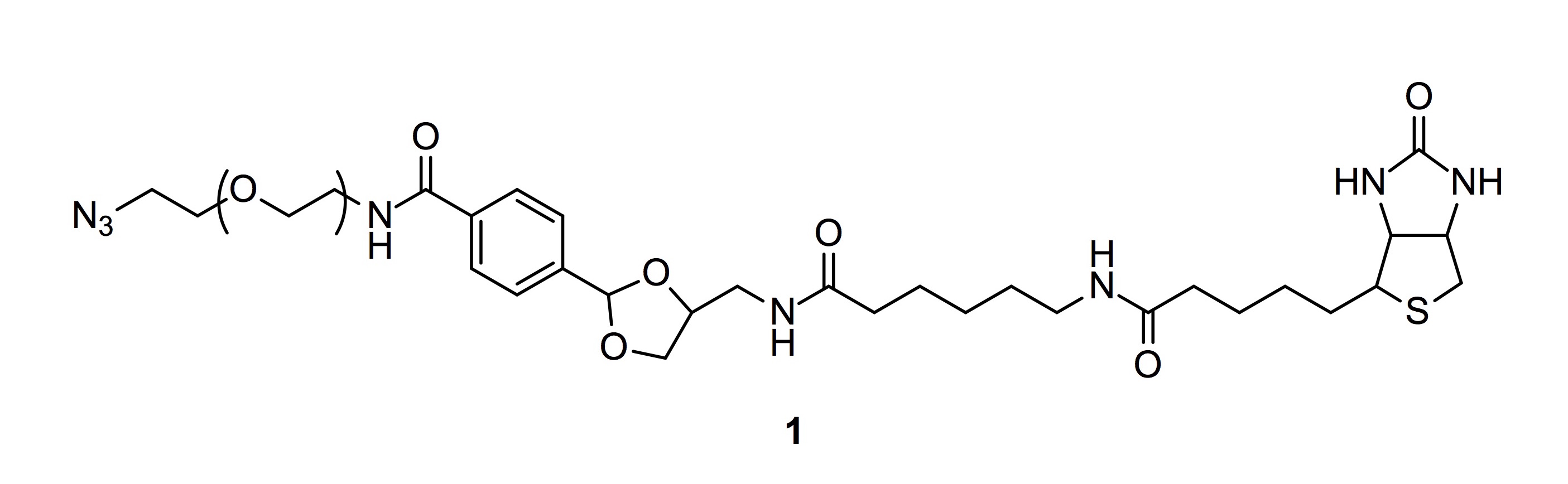
Lee, S., and Sampson, N. S. (2017) “A cleavable cyclic acetal for affinity capture, release, and conjugation”, in press, Methods in Molecular Biology.
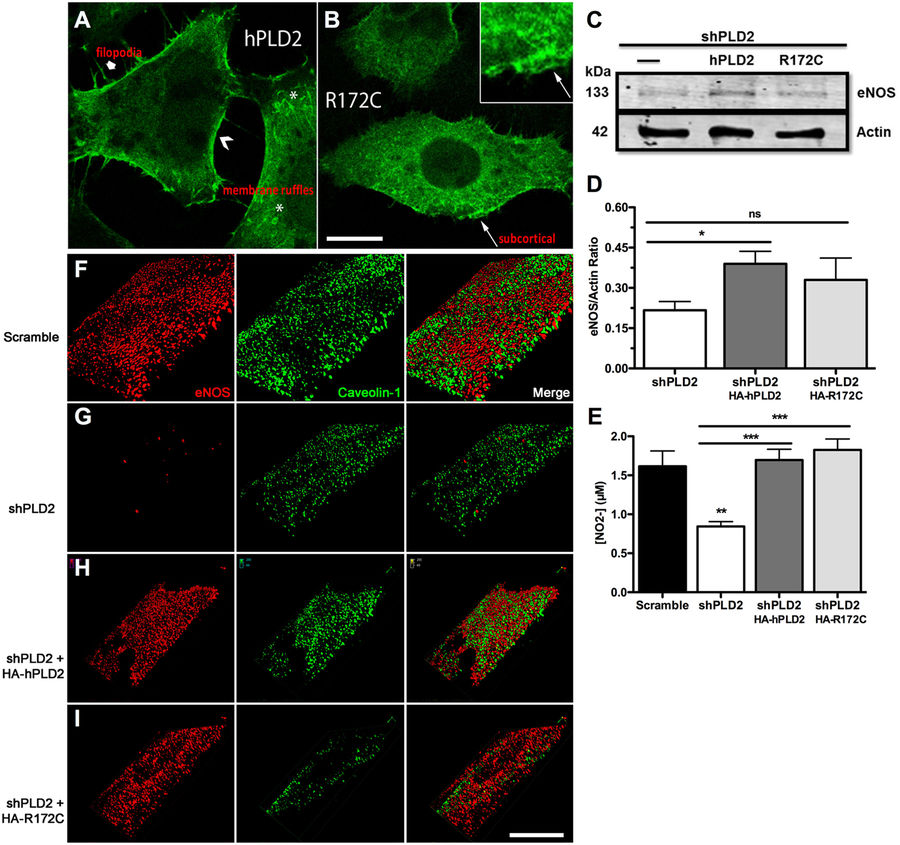
Nelson, R. K., Ya-Ping, J., Gadbery, J., Abedeen, D., Sampson, N.S., Lin, R. Z., and Frohman, M. (2017) “Phospholipase D2 loss results in increased blood pressure via inhibition of the eNOS pathway,” Scientific Reports, 7, 9112, DOI: 10.1038/s41598-017-09852-4.
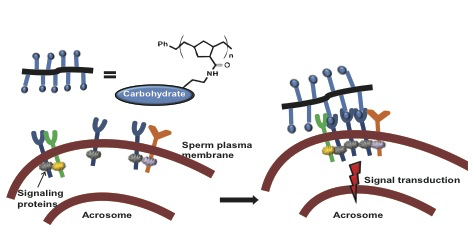
Huang, H., Rodolis, M. T., Bhatia, S. R., and Sampson, N. S. (2017) “Sugars require rigid multivalent displays for activation of mouse sperm acrosomal exocytosis,” Biochemistry, DOI: 10.1021/acs.biochem.7b00166.
Rodolis, M., Huang, J., and Sampson, N. S. (2016) “Glycopolymer Induction of Mouse Sperm Acrosomal Exocytosis Shows Highly Cooperative Self-Antagonism,” 474, 435-440. , DOI: 10.1016/j.bbrc.2016.05.003.
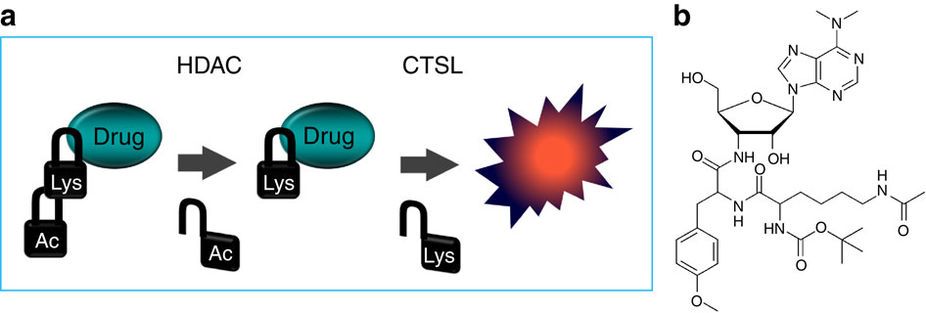
Charkoudian, L. K., Sampson, N. S., Kumar, K. and Kritzer, J. A. (2016) “Designing convergent chemistry curricula,” Nature Chemical Biology, 12, 382–386. doi:10.1038/nchembio.2090 – Commentary Ueki, N., Wang, W., Swenson, C., McNaughton, C., Sampson, N. S., and Hayman, M. J. (2016) “Synthesis and Preclinical Evaluation of a Highly Improved Anticancer Prodrug Activated by Histone Deacetylases and Cathepsin L,” Theranostics, 6, 808-816. DOI:10.7150/thno.1382
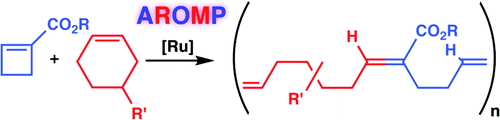
Parker, K. A., Sampson, N. S. (2016) “From ROMP to AROMP: using substituted cyclobutenes to precisely control polymer structure,” Accounts of Chemical Research. 49, 408-417. DOI: 10.1021/acs.accounts.5b00490.
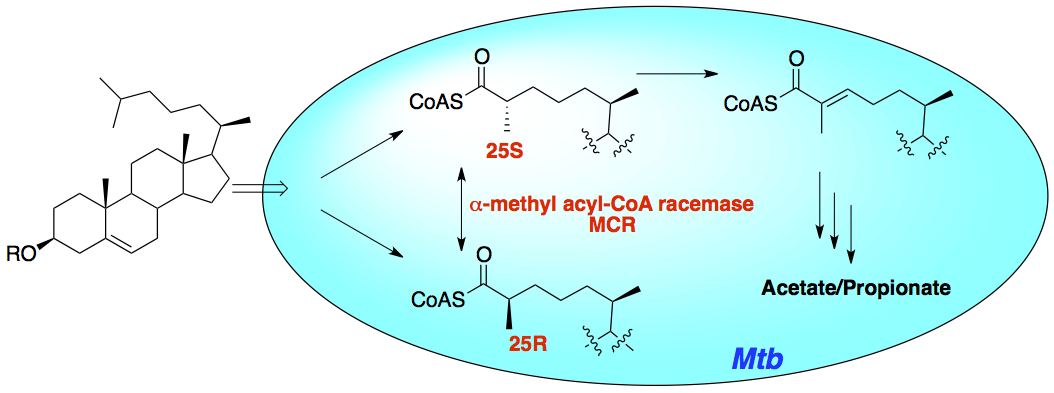
Lu, R, Schmitz, W., and Sampson, N. S. (2015) “α-Methyl Acyl CoA racemase provides Mycobacterium tuberculosis catabolic access to cholesterol esters,” Biochemistry, 54, 5669-5672. DOI: 10.1021/acs.biochem.5b00911
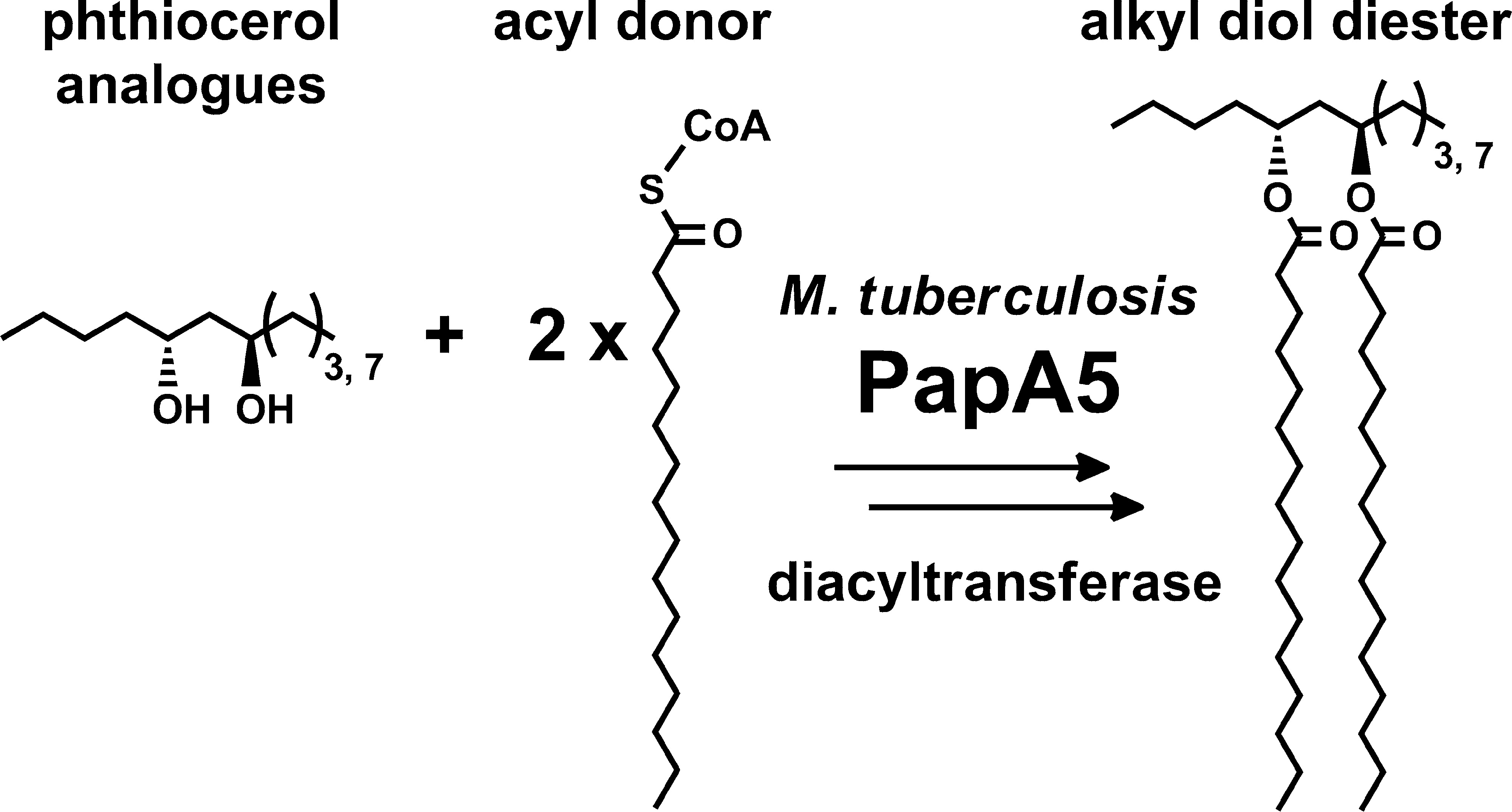
Touchette, M. H., Bommineni, G. R., Delle Bovi, R. J., Gadbery, J. E., Nicora, C. D., Shukla, A. K., Kyle, J. E., Metz, T. O., Martin, D. W., Sampson, N. S., Miller, W. T., Tonge, P. J., and Seeliger, J. C. (2015) “Diacyltransferase activity and chain length specificity of Mycobacterium tuberculosis PapA5 in the synthesis of alkyl β-diol lipids,” Biochemistry, 54, 5457-5468. DOI: 10.1021/acs.biochem.5b00455
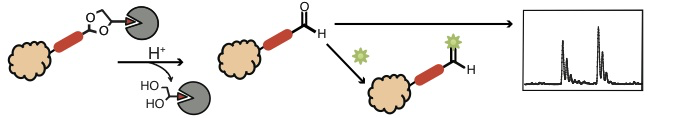
Lee, S., Wang, W., Lee, Y. and Sampson, N. S. (2015) “Cyclic acetals as cleavable linkers for affinity capture,” Organic and Biomolecular Chemistry, 13, 8445-8452. DOI: 10.1039/C5OB01056J
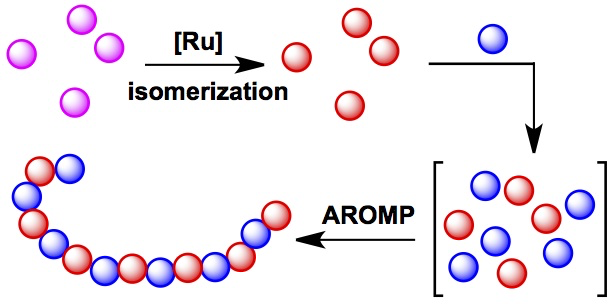
Tan, L., Li, G., Parker, K. A. and Sampson, N. S. (2015) “Ru-catalyzed isomerization provides access to alternating copolymers via ring-opening metathesis polymerization,” Macromolecules, 48, 4793-4800. DOI: 10.1021/acs.macromol.5b01058
Yang, M., Lu, R. Guja, K. E., Wipperman, M., St. Clair, J. Bonds, A., Garcia-Diaz, M. And Sampson, N. S. (2015) “Unraveling cholesterol catabolism in Mycobacterium tuberculosis: the ChsE4-ChsE5 α2β2 acyl-CoA dehydrogenase initiates β-oxidation of 3-oxo-cholest-4-en-26-oyl CoA,” ACS Infectious Disease, 1, 110-125. DOI: 10.1021/id500033m
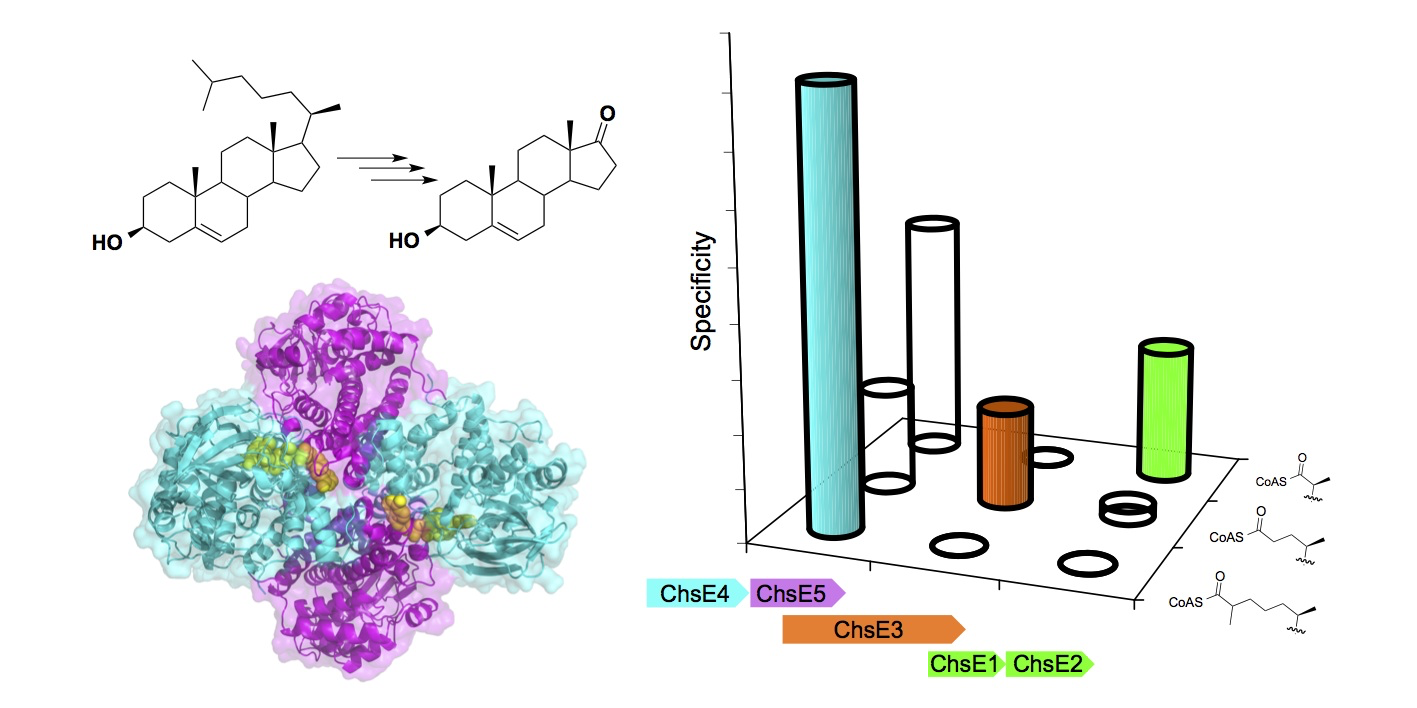
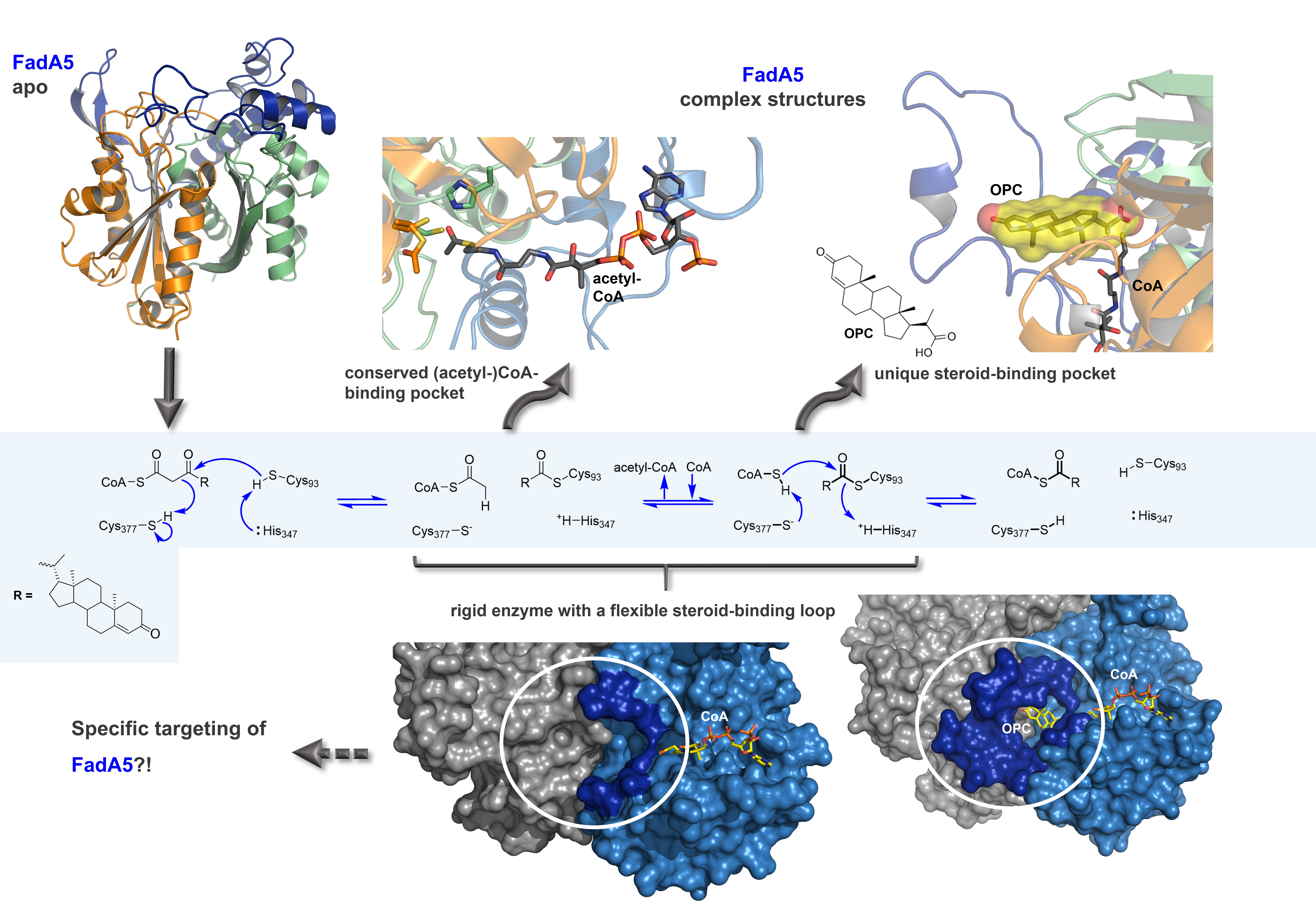
Schaefer, C., Lu, R., Nesbitt, N. M., Schiebel, J. Sampson, N. S., and Kisker, C. (2015) “FadA5 a thiolase from Mycobacterium tuberculosis – a unique steroid-binding pocket reveals the potential for drug development against tuberculosis,” Structure, 23, 21-33. DOI: 10.1016/j.str.2014.10.010See Commentary
Yang, M., Guja, K. E., Thomas, S. T., Garcia-Diaz, M. And Sampson, N. S. (2014) “A distinct MaoC-like enoyl-CoA hydratase architecture mediates cholesterol catabolism in Mycobacterium tuberculosis,” ACS Chemical Biology,9, 2632-2645 DOI: 10.1021/cb500232h
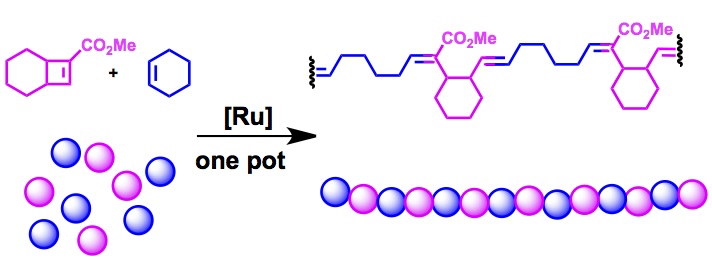
Tan, L., Parker, K., and Sampson, N. S. (2014) “A Bicyclo[4.2.0]octene-derived monomer provides completely linear alternating copolymers via alternating ring-opening metathesis polymerization (AROMP),” Macromolecules, 47, 6572-6579 DOI: 10.1021/ma5012039
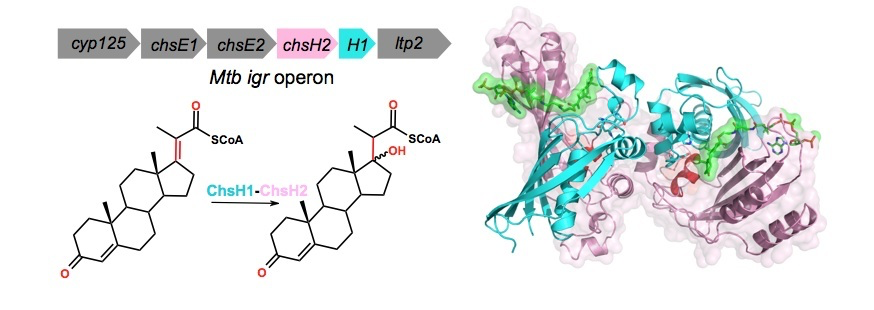
Wipperman, M., Sampson, N. S., and Thomas, S. T. (2014) “Pathogen “Roid Rage”: Cholesterol Utilization by Mycobacterium tuberculosis,”CRC Critical Reviews in Biochemistry, 49, 269-293 DOI 10.3109/10409238.2014.895700.
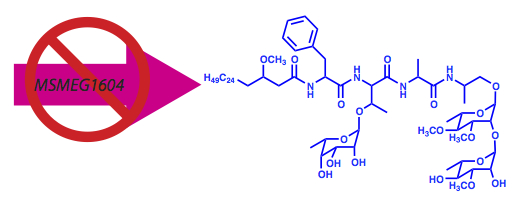
Gao, J. and Sampson, N. S. (2014) “A GMC Oxidoreductase Homolog is Required for Acetylation of Glycopeptidolipid in Mycobacterium smegmatis,” Biochemistry53, 611-613 DOI: 10.1021/bi4015083
Wu, L., and Sampson, N. S. (2014) “Fucose, Mannose and β-N-Acetylglucosamine Glycopoly-mers Initiate the Mouse Sperm Acrosome Reaction Through Convergent Signaling Pathways,” ACS Chem. Biol.9, 468-475 DOI: 10.1021/cb400550j
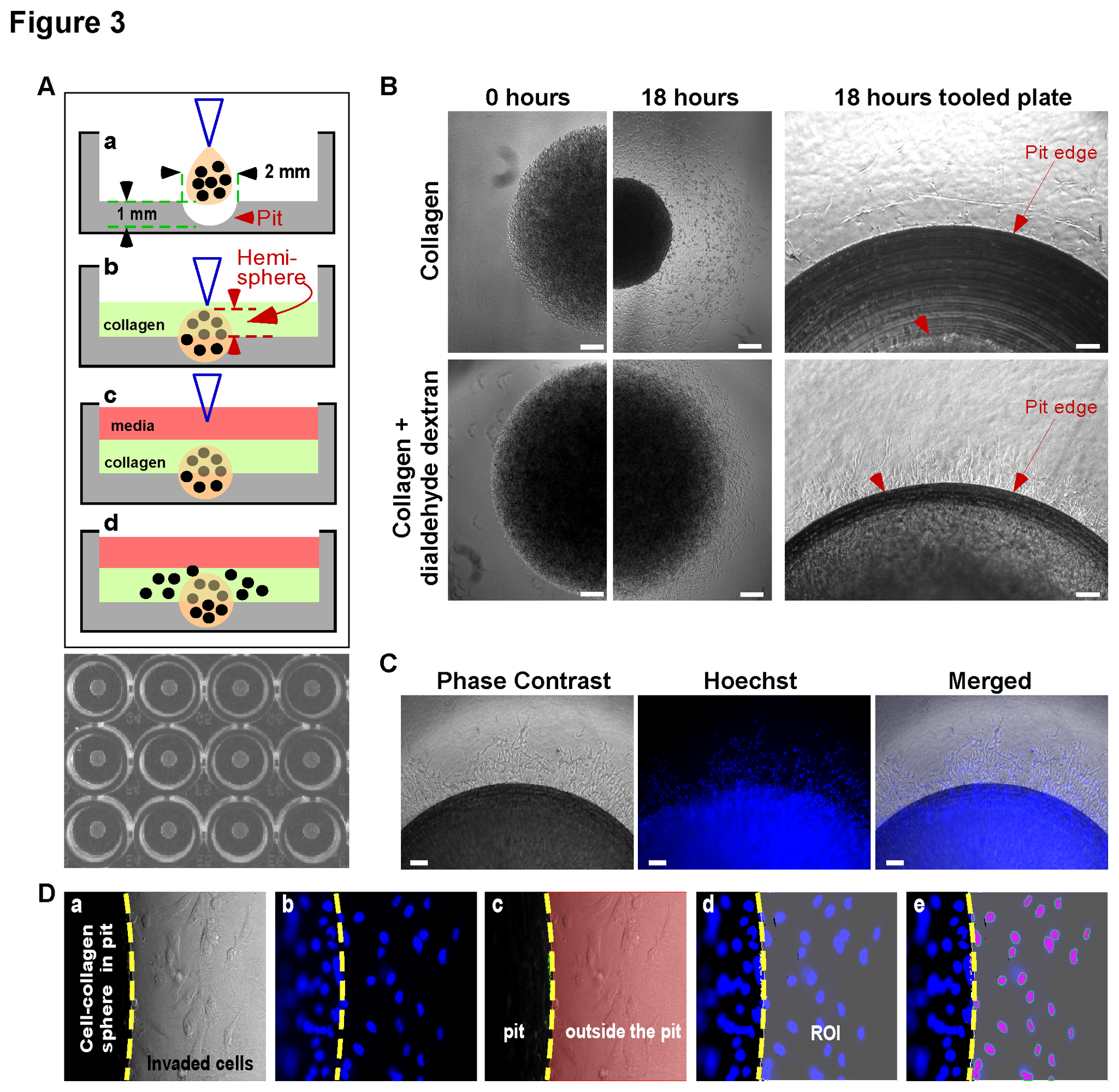
Evensen N.A., Li J., Yang J., Yu X., Sampson N.S., Zucker, S., Cao, J. (2013) “Development of A High-throughput Screening Invasion Assay for Anti-Cancer Drug Discovery,” PLoS One, 8(12): e82811. DOI: 10.1371/journal.pone.0082811
Ueki, N. Lee, S. Sampson, N. S., Hayman, M. J. (2013) “Selective cancer targeting with prodrugs activated by histone deacetylase and tumour-associated protease,” Nature Comm., 4, 2735-2742. DOI: 10.1038/ncomms3735
Commentary: New Scientist Genetic Engineering and Biotech News
Romulus, J., Tan, L., Weck, M., Sampson, N. S. (2013) “Alternating ROMP copolymers containing charge-transfer units,” ACS Macro Letters, 2, 749-752. DOI: 10.1021/mz4002673
Wipperman, M.F., Yang, M., Thomas, S.T., Sampson, N.S. (2013) “Shrinking the FadE proteome of Mycobacterium tuberculosis: Insights into cholesterol metabolism through identification of an α2β2 heterotetrameric acyl-CoA dehydrogenase family,” J Bacteriol. 195 (19): 4331-41. DOI: 10.1128/JB.00502-13 See commentary at DOI: 10.1128/JB.00867-13
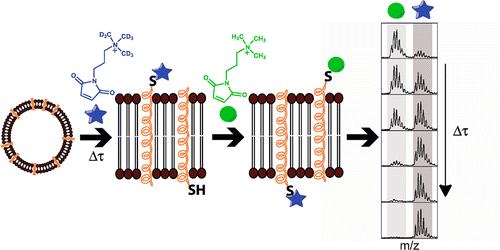
Su, C.Y., London, E., Sampson, N.S. (2013) “Mapping peptide thiol accessibility in membranes using a quaternary ammonium isotope-coded mass tag (ICMT),” Bioconjug Chem. 24 (7), pp 1235–1247. DOI: 10.1021/bc400171j
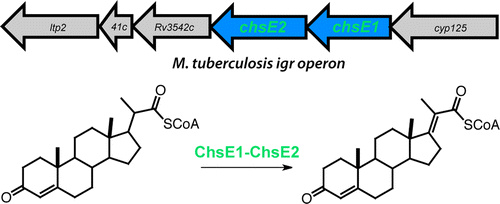
Thomas, S.T., Sampson, N.S. “Mycobacterium tuberculosis utilizes a unique heterotetrameric structure for dehydrogenation of the cholesterol side chain,” Biochemistry, (2013), 52, 17, 2895-904. DOI: 10.1021/bi4002979
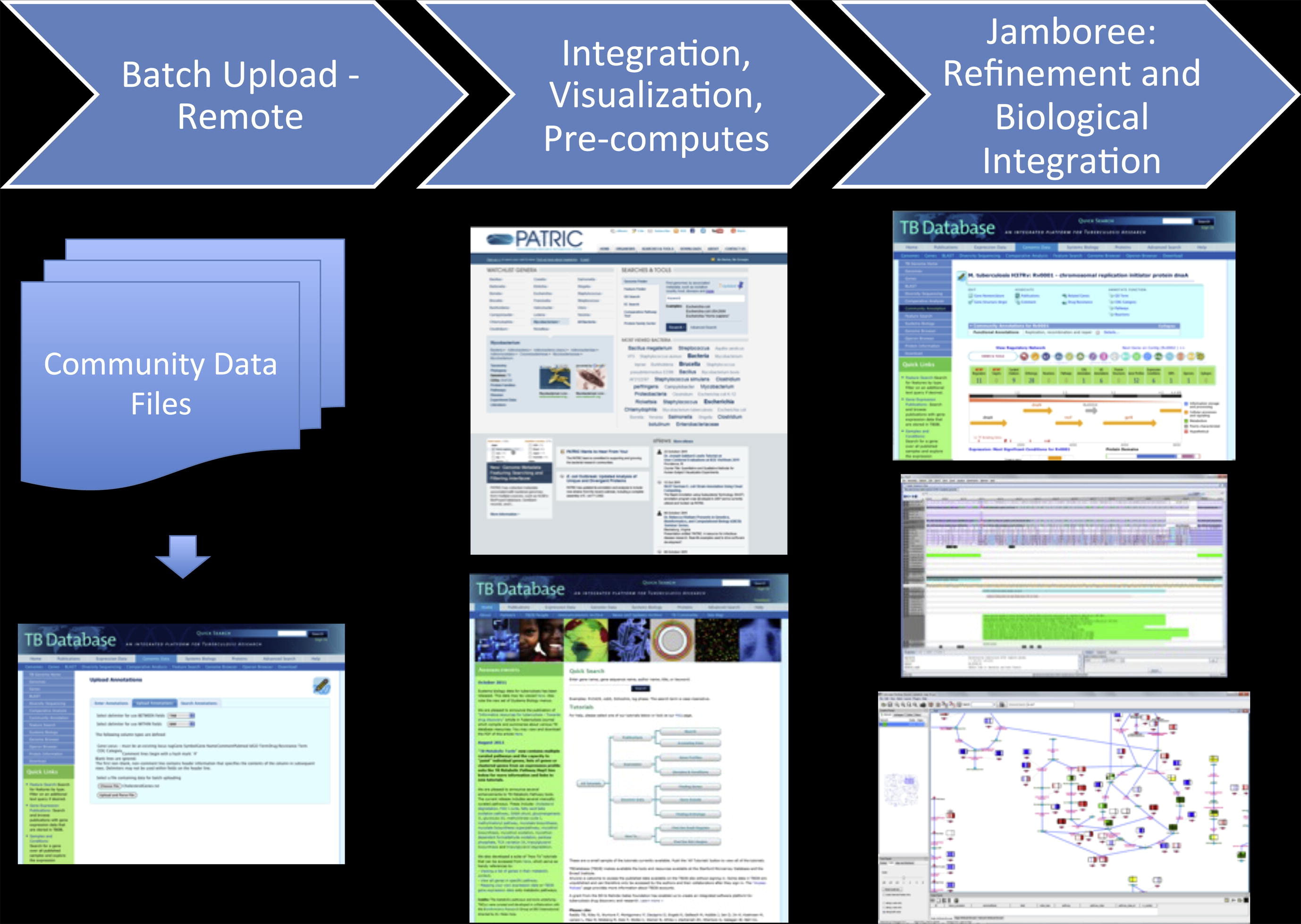
Slayden, R.A., Jackson, M., Zucker, J., Ramirez, M.V., Dawson, C.C., Crew, R., Sampson, N.S., Thomas, S.T., Jamshidi, N., Sisk, P., Caspi, R., Crick, D.C., McNeil, M.R., Pavelka, M.S., Niederweis, M., Siroy, A., Dona, V., McFadden, J., Boshoff, H., Lew, J.M. (2013) “Updating and curating metabolic pathways of TB,” Tuberculosis, 93, 1, 47-59. DOI: 10.1016/j.tube.2012.11.001
Thomas, S.T., VanderVen, B.C., Sherman, D.R., Russell, D.G., Sampson, N.S. “Pathway profiling in Mycobacterium tuberculosis: elucidation of cholesterol-derived catabolite and enzymes that catalyze its metabolism,” J. Biol. Chem, (2011), 286, 51, 43668-78. DOI: 10.1074/jbc.M111.313643
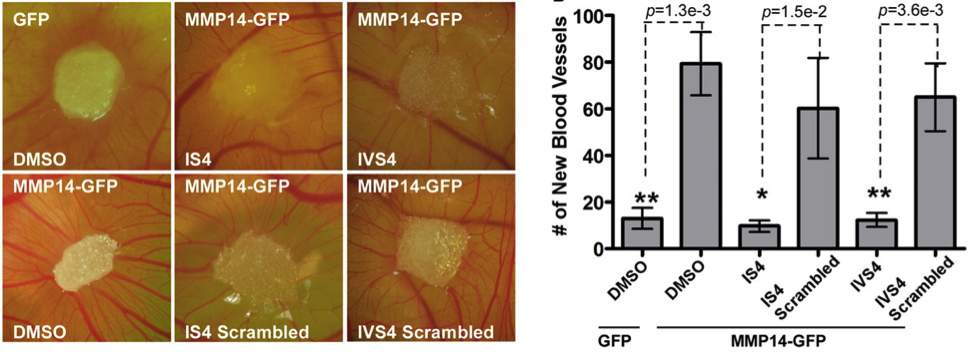
Zarrabi, K.; Dufour, A.; Li, J; Kuscu, C.; Kozarekar, P.; Zhi, J.; Sampson, N. S.; Zucker, S.; Cao, J. (2011) “Inhibition of matrix metalloproteinase-14 (MMP-14)-mediated cancer cell migration,” J. Biol. Chem., 286, 38, 33167-77. DOI: 10.1074/jbc.M111.256644
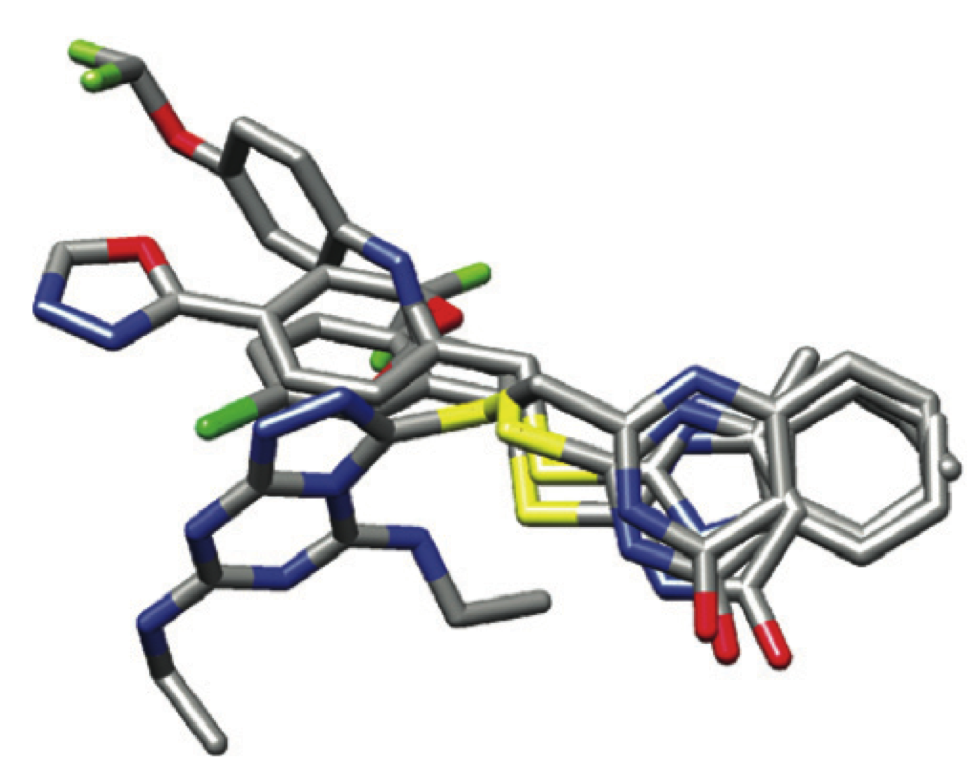
Dufour, A.; Sampson, N. S.; Rizzo, R; DeLeon, J; Li, J; Kuscu, C.; Zhi, J.; Jaber, N.; Liu, E.; Zucker, S.; Cao, J. (2011) “Small molecule anti-cancer therapy selectively targets the hemopexin domain of matrix metalloproteinase-9 (MMP-9),” Cancer Res., 71, 4977-4988. DOI: 10.1158/0008-5472.CAN-10-4552
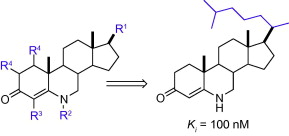
Thomas, S.; Yang, X.; Sampson, N. S. (2011), “Inhibition of the M. tuberculosis 3Beta-Hydroxysteroid Dehydrogenase by Azasteroids,” Bioorg. Med. Chem. Lett., 21, 2216-2219. DOI: 10.1016/j.bmcl.2011.03.004
Song, A.; Walker, S. G.; Parker, K. A.; Sampson, N. S. (2011) “Antibacterial studies of alternating, random and homo-polymers with varied positions of cationic side chains,” ACS Chem. Biol., 6, 590-599. DOI: 10.1021/cb100413w
Yang, X.; Gao, J.; Smith, I.; Dubnau, E.; Sampson, N. S. (2011), “Cholesterol Is not an essential source of nutrition for Mycobacterium tuberculosis during infection,” J. Bact., 193, 1473-147. DOI: 10.1128/JB.01210-10
Dufour, A., Sampson, N. S., Kuscu, C., Zucker, S. and Cao, J. (2010) “Roles of MMP-9 homodimer and heterodimer in cell migration,”J. Biol. Chem., 285, 35944-56. DOI: 10.1074/jbc.M109.091769
Song, A.; Parker, K. A.; Sampson, N. S. (2010) “Cyclic alternating ROMP (CAROMP). Rapid access to functionalized cyclic polymers,”Org. Lett. 12, 3729-3731. DOI: 10.1021/ol101432m
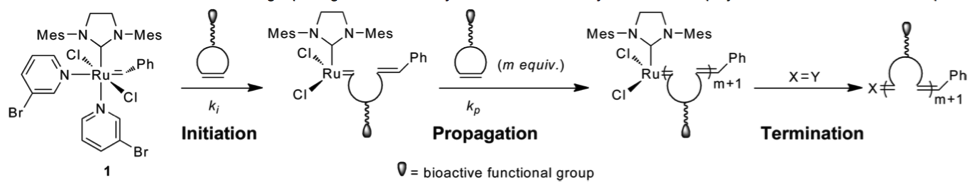
Song, A.; Lee, J.; Parker, K. A.; Sampson, N. S. (2010) “Scope of the ring opening metathesis polymerization (ROMP) reaction of 1-substituted cyclobutenes,” J. Am. Chem. Soc., 132, 10513–10520. DOI: 10.1021/ja1037098
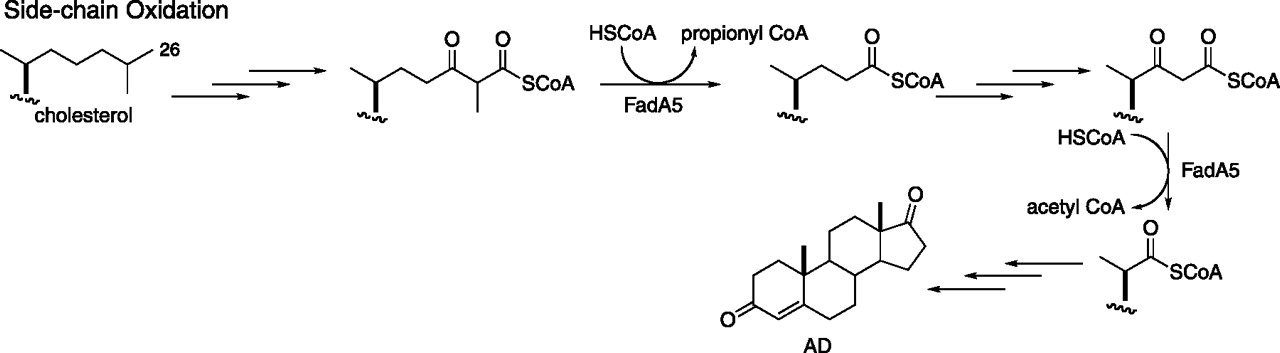
Nesbitt, N. M.*; Yang, X.*; Fontán, P.; Kolesnikova, I.; Smith, I.; Sampson, N. S.*; Dubnau, E.* (2010) “A thiolase of M. tuberculosis is required for virulence and for production of androstenedione and androstadienedione from cholesterol,” Infect. Immun., 78. 275-282. *co-authors DOI: 10.1128/IAI.00893-09
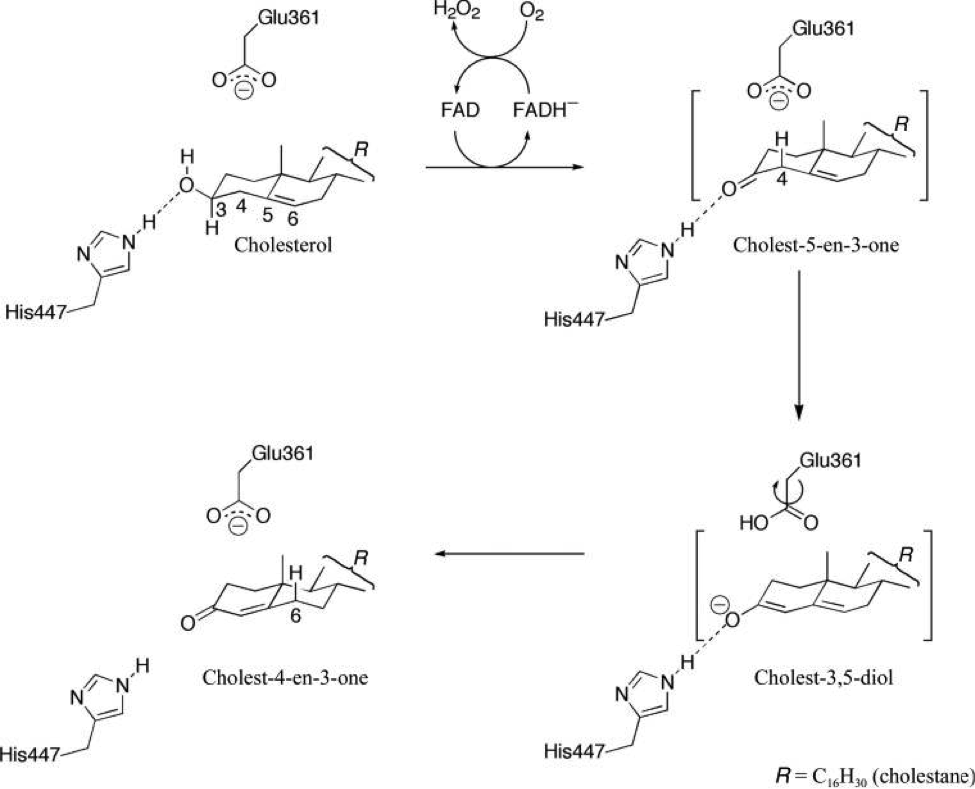
Lyubimov, A. Y.; Chen, L.; Sampson, N. S.; Vrielink, A. (2009) “A hydrogen-bonding network is important for oxidation and isomerization in the reaction catalyzed by cholesterol oxidase,” Acta Crys. D, 65, 1222-1231. DOI: 10.1107/S0907444909037421
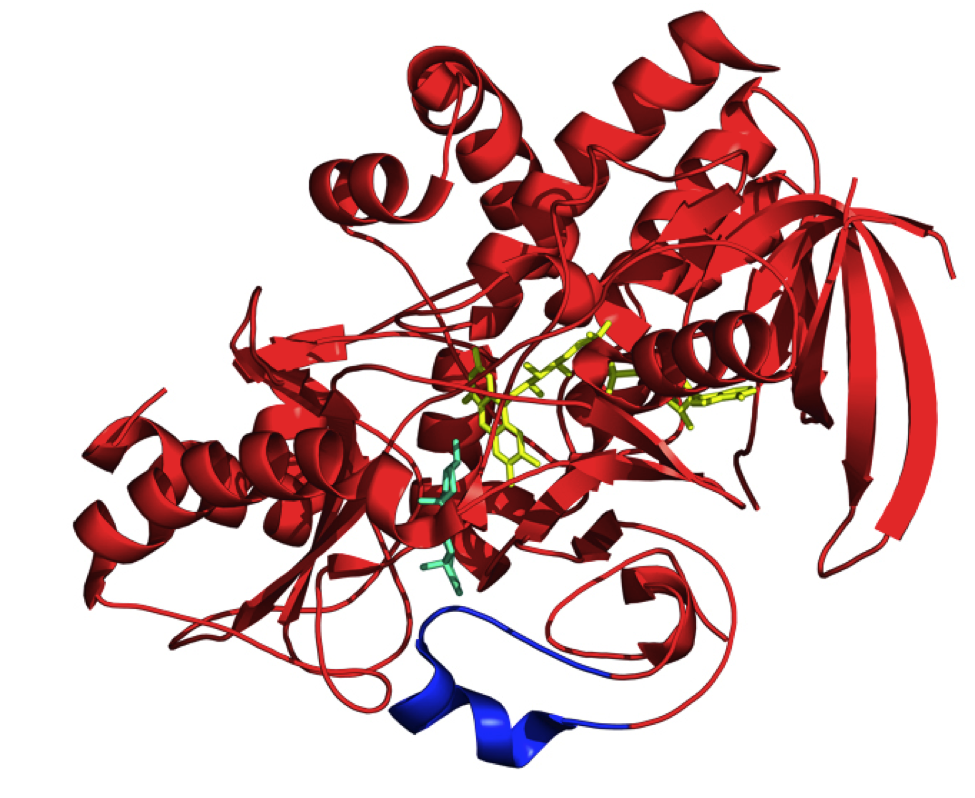
Kreit, J; Sampson, N. S. (2009) “Cholesterol oxidase: Physiological functions,” FEBS,276, 6844-6856. DOI: 10.1111/j.1742-4658.2009.07378.x
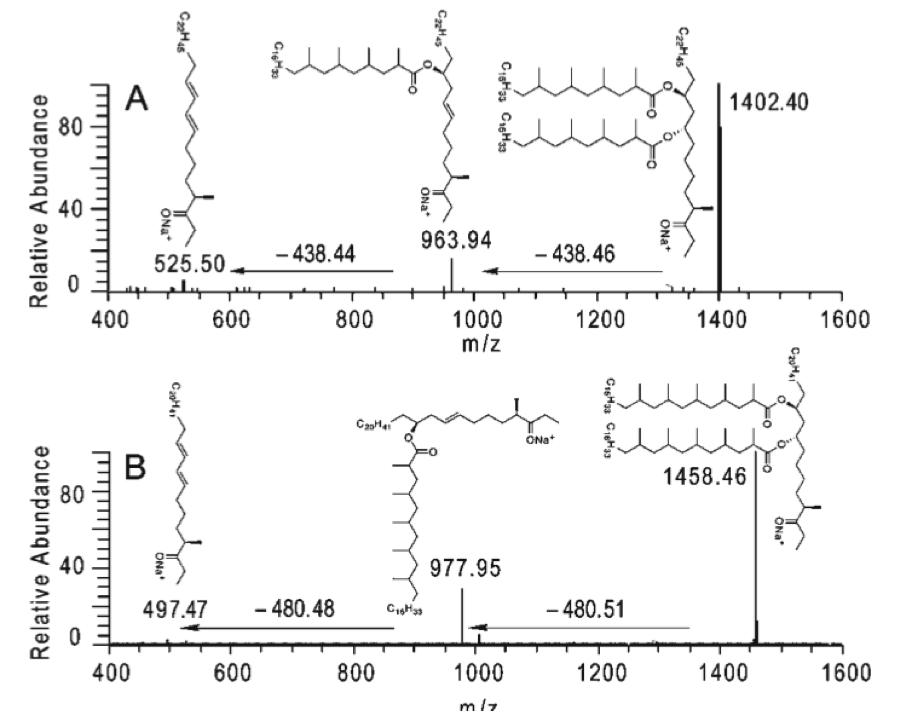
Yang, X.; Nesbitt, N. M.; Dubnau, E.; Smith, I.; Sampson, N. S. (2009), “Cholesterol metabolism increases the metabolic pool of propionate in M. tuberculosis,” Biochemistry, 48, 3819-3821. DOI: 10.1021/bi9005418
Baessler, K.; Lee, Y.; Sampson, N. S. (2009) “Beta1 Integrin is an adhesion protein for sperm binding to eggs,” ACS Chem. Biology, 4, 357-366. DOI: 10.1021/cb900013d
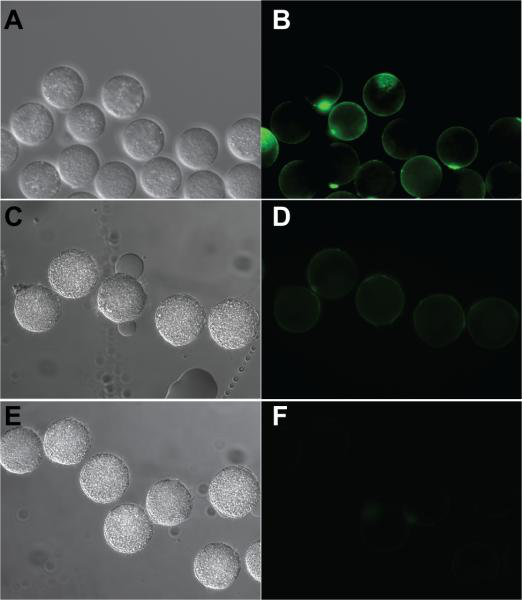
Song, A..; Parker, K.*; Sampson, N. S.* (2009) “Synthesis of copolymers by alternating romp (AROMP),” J. Am. Chem. Soc., 131, 3444–3445. DOI: 10.1021/ja809661k
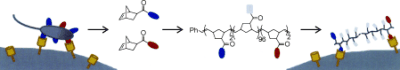
Lee, Y.; Sampson, N. S. (2009) “Polymeric ADAM protein mimics interrogate mammalian sperm-egg binding,” ChemBioChem, 10, 929-937. DOI: 10.1002/cbic.200800791
Dufour, A.; Sampson, N. S.; Zucker, S.; Cao, J. (2008) “Role of the hemopexin domain of matrix metalloproteinase-9 in cell migration,” J. Cell Physiol., 217, 643-651. DOI: 10.1002/jcp.21535
Elalami, A; Baessler, K.; Kong, F.; Sampson, N. S.; Kreit, J. (2008) “Subcellular forms of cholesterol oxidase from Rhodococcus sp. CIP 105 335: induction, solubilization and characterization,” in Current Research Topics in Applied Microbiology and Microbial Biotechnology, World Scientific Publishing.
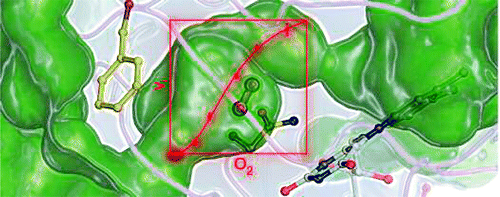
Chen, L.; Lyubimov, A. Y.; Vrielink, A.; Sampson, N. S. (2008) “The binding and release of oxygen and hydrogen peroxide are directed by a hydrophobic tunnel in cholesterol oxidase.,” Biochemistry, 47, 5368–5377. DOI: 10.1021/bi800228w
Sampson, N. S. and Kwak, S. (2008) “ Catalysis at the membrane Interface: Cholesterol oxidase as a case study“, in Proceedings of the 3rd International Beilstein Workshop on Experimental Standard Conditions of Enzyme Characterizations. Beilstein, Germany. DOI: n/a
Yang, X; Dubnau, E. Smith, I; Sampson, N. S. (2007) “Rv1106c from Mycobacterium tuberculosis is a 3b-hydroxysteroid dehydrogenase,”Biochemistry,46, 9058-9067. DOI: 10.1021/bi700688x
Nesbitt, N. M.; Sampson, N.S. (2007) “Antifungal Tradecraft by Cholesterol Oxidase,” Chem. Biol. 14, 238-241. DOI: 10.1016/j.chembiol.2007.03.003
Lyubimov, A. Y.; Heard, K. Tang, H.; Sampson, N. S; Vrielink, A. (2007) “Distortion of flavin geometry linked to ligand binding in cholesterol oxidase,” Protein Science, 16, 2647-2656. PMC2222809
Kempf, J. G.; Jung, J-y.; Ragain, C.; Sampson, N. S.; Loria, J. P. (2007) “Dynamic requirements for a functional protein hinge,” J. Mol. Biol.368, 131-149. DOI: 10.1016/j.jmb.2007.01.074
Lee, Y.; Baessler, K.; Sampson, N. S. (2006) “ROMP of norbornyl oligopeptides: A versatile synthetic method for exploring receptor topology,” in Understanding Biology Using Peptides, S. Blondelle, ed., American Peptide Society (2005), 59-60. DOI:
Lee, Y.; Sampson, N. S. (2006) “ROMPing the Cellular Landscape: Linear Scaffolds for Molecular Recognition,” Curr. Opin. Struct. Biol. 16, 544-550. DOI: 10.1016/j.sbi.2006.05.015
Lee, J; Parker, K. L.*; Sampson, N. S.* (2006) “Amino Acid-Bearing ROMP polymers with a Stereoregular Backbone,” J. Am. Chem. Soc. 128, 4578-4579. DOI: 10.1021/ja058801v
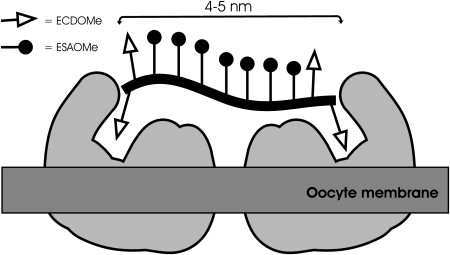
Baessler, K.; Lee, Y.; Roberts, K. S.; Facompre, N.; Sampson, N. S. (2006) “Multivalent fertilin beta oligopeptides: the dependence of fertilization inhibition on length and density,” Chem. Biol. 13, 251-259. DOI: 10.1016/j.chembiol.2005.12.010
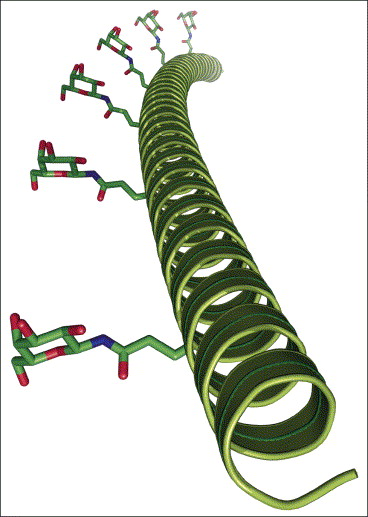
Lee, Y.; Sampson, N. S.; (2006) “ROMPing the Cellular Landscape: Linear Scaffolds for Molecular Recognition,” Curr. Opin. Struct. Biol. 16, 544-550. DOI: 10.1016/j.sbi.2006.05.015
Baessler, K.; Lee, Y.; Sampson, N.S. (2005) “ROMP of norbonyl oligopeptides: A versatile synthetic method for exploring receptor topology, “in Understanding Biology Using Peptides, S. Blondelle, ed. American Peptide Society,59-60. DOI:
Roberts, S. K.; Sampson, N. S. (2004) “A Facile Synthetic Route Leading to Fluorescently Labeled ROMP Polymers,” Org. Lett., 6, 3253-3255. DOI: 10.1021/ol048935y
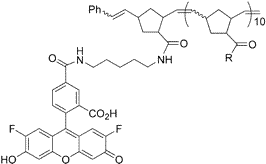
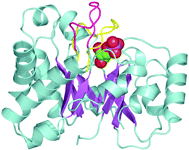
Xiang, J.; Jung, J-y.; Sampson, N. S. (2004) “Entropy Effects on Protein Hinges: the Reaction Catalyzed by Triosephosphate Isomerase,”Biochemistry, 43, 11436-11445. DOI: 10.1021/bi049208d
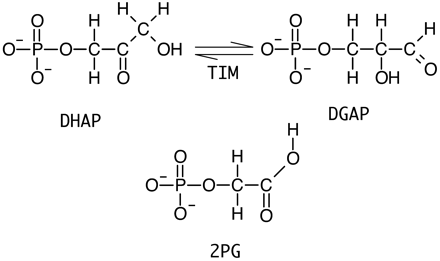
Kursula, I.; Salin, M.; Sun, J.; Borledge, B.; Haapalainen, A.; Sampson, N. S.; Wierenga, R. K. (2004) “Understanding Protein Lids: Structural Analysis of Active Hinge Mutants in Triosephosphate Isomerase,” Prot. Engineering, Design Selection, 17, 375-382. DOI: 10.1093/protein/gzh048
Xiang, J.; Sampson, N. S. (2004) “Library Screening Studies to Investigate Substrate Specificity in the Reaction Catalyzed by Cholesterol Oxidase,” Prot. Engineering, Design Selection, 17, 341-348. DOI: 10.1093/protein/gzh041
Konkar, S.; Gupta, S.; Sampson, N. S. (2004) “Fertilinb Liposomes Inhibit In Vitro Fertilization by Steric Stabilization,” Bioorg. Med. Chem. Lett. 4, 1381-1384. DOI:
Ahn, K-w.; Sampson, N. S. (2004) “Cholesterol Oxidase Senses Subtle Changes in Lipid Structure,” Biochemistry 43, 827-836. DOI: 10.1021/bi035697q
Vrielink, A.; Sampson, N. S.; (2003) “Sub-Ångstrom Resolution Protein Structures: Is Seeing Believing?,” Curr. Opin. Struct. Biol. 13, 709-713. DOI: 10.1016/j.sbi.2003.10.012
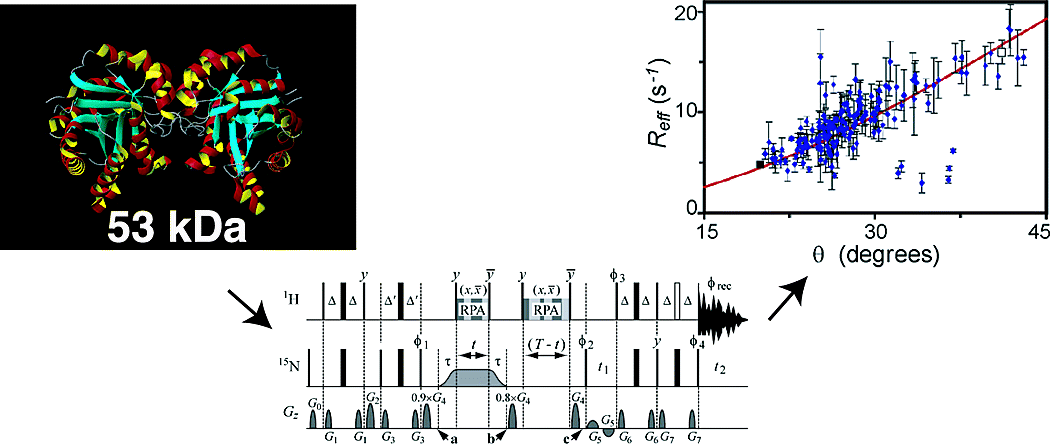
Kempf, J. G.; Jung, J-y.; Sampson, N. S.; Loria, J. P. (2003) Off-Resonance TROSY (R1p-R1) for Quantitation of Fast Exchange Processes in Large Proteins,” J. Am. Chem. Soc. 125, 12064-12065. DOI: 10.1021/ja037101s
Sampson, N. S.; Vrielink, A. (2003) “Cholesterol Oxidases: A Study of Nature’s Approach to Protein Design,” Acc. Chem. Res 36, 713-722. DOI: 10.1021/ar9800587
Roberts, S. K.; Konkar, S.; Sampson, N. S. (2003) “Comparison of Fertilinb Peptide-Substituted Polymers and Liposomes as Inhibitors ofIn Vitro Fertilization,” ChemBioChem 4, 1229-1231. DOI: 10.1002/cbic.200300672
Lario, P.; Sampson, N.S.; Vrielink, A. (2003) “Sub-atomic resolution crystal structure of cholesterol oxidase: What atomic resolution crystallography reveals about enzyme mechanism and the role of the FAD cofactor in redox activity,” J. Mol. Biol. 326 1635-1650. DOI:
Roberts, S.K.; Sampson, N. S. (2003) “Increased Polymer Length of Oligopeptide-substituted Polynorbornenes using LiCl,” J. Org. Chem.68, 2020-2023. DOI:
Ye, Y.; Liu, Pingsheng; Anderson, R.; Sampson, N. S. (2002) “Construction of a Catalytically Inactive Cholesterol Oxidase Mutant: Investigation of the Interplay Between Active Site Residues Glutamate 361 and Histidine 447,” Arch. Biochem Biophys. 402, 235-242. DOI:
Li, H.; Sampson, N. S. (2002) “Structural Analysis of Cyclic Peptide Fertilinb Mimics That Are Ligands for a6b1 Integrin,” J. Pep. Res. 59, 49-54. DOI:
Gupta, S.; Sampson, N. S. (2001) “Dimyristoylated Peptides Incorporated into Lipsomes Are Polyvalent Fertilinb Mimics,” Org. Lett. 3, 3333-333. DOI:
Sampson, N. S. (2001) “The Emerging Global Paradigm for Scientific Research,” in New Voices in Chemistry, Chem. Eng. News, 26 Mar 2001, p. 187. DOI:
Ye, Y.; Lario, P. Vrielink, A.; Sampson, N. S. (2001) “Structural and Kinetic Analysis of the Role of Asn485 in the Reaction Catalyzed by Cholesterol Oxidase,” Biochemistry, 40, 13779-13787. DOI:
Sampson, N.S.; Mrksich, M.; Bertozzi, C.R. (2001) “Surface Molecular Recognition,” Proc. Nat. Acad. Sci. U.S.A. 98, 12870-12871. DOI:
Sampson, N. S.; Sarah T. Ryan; Deborah A. Enke; Dominic Cosgrove; Victor Koteliansky; Philip Gotwals (2001) “Global Gene Expression Analysis Reveals a Role for the a1Integrin in Renal Pathogenesis,” J. Biol. Chem, 276 34182-34188. DOI:
Xiang, J; Sun, J.; Sampson, N. S. (2001) “The Importance of Hinge Sequence for Loop Function and Catalytic Activity in the Reaction Catalyzed by Triosephosphate Isomerase,” J. Mol. Biol, 307, 1103-1112. DOI:
Sampson, N. S. (2001) “Dissection of a Flavo-Enzyme Active Site: the Reaction Catalyzed by Cholesterol Oxidase,” Antioxidants and Redox Signalling, 3, 839-846. DOI:
Chen, X.; Wolfgang, D.; Sampson, N. S., (2000) “Use of the Parallax-Quench Method to Determine the Position of the Active-Site Loop of Cholesterol Oxidase in Lipid Bilayers,” Biochemistry, 39, 13383-13389. DOI:
McCann, A.; Sampson, N. S., (2000) “A C6-FAD Adduct is Formed Upon Irreversible Inactivation of Cholesterol Oxidase by 2a,3a-Cyclopropano-5a-cholestan-3b-ol,” J. Am.Chem. Soc., 122, 35-39. DOI:
Gupta, S.; Li, H.; Sampson, N. S., (2000) “Characterization of Fertilinb-Disintegrin Binding Specificity in Sperm-Egg Adhesion,” Bioorg. Med. Chem., 8, 723-729. DOI:
Sun, J.; Sampson, N. S., (1999) “Understanding Protein Lids: Kinetic Analysis of Active Hinge Mutants in Triosephosphate Isomerase,”Biochemistry, 38, 11474-11481. DOI:
Yue, Q. K.; Kass, I. J.; Sampson, N. S.; Vrielink, A., (1999) “Crystal Structure Determination of Cholesterol Oxidase from Streptomycesand Structural Characterization of Key Active Site Mutants,” Biochemistry, 38, 4277-4286. DOI:
Chen, H.; Sampson, N.S., (1999) “Mediation of Mammalian Sperm-Egg Fusion: Evidence That Mouse Egg a6b1 Integrin is the Receptor for Sperm Fertilinb,” Chem. Biol., 6, 1-10. DOI:
Kass, I. J.; Sampson, N. S., (1998) “Evaluation of the Role of His447 in the Reaction Catalyzed by Cholesterol Oxidase,”Biochemistry, 37, 17990-18000. DOI:
Chen, H.; Pyluck, A.; Janik, M.; Sampson, N. S., (1998) “Peptides Corresponding to the Epidermal Growth Factor-like Domain of Mouse Fertilin: Synthesis and Biological Activity,” Biopolymers (Peptide Science), 47, 299-307. DOI:
Kass, I. J.; Sampson, N. S., (1998) “The Importance of Glu361 Position in the Reaction Catalyzed by Cholesterol Oxidase,” Bioorg. Med. Chem. Lett., 8, 2663-2668. DOI:
Sun, J.; Sampson, N. S., (1998) “Determination of the Amino Acid Requirements for a Protein Hinge in Triosephosphate Isomerase,”Prot. Science, 7, 1495-1505. DOI:
Sampson, N. S.; Kass, I. J.; Ghoshroy, K. B., (1998) “A Truncated W Loop Mutant of Cholesterol Oxidase Has Altered Substrate Specificity,” Biochemistry, 37, 5770-5778. DOI:
Sampson, N. S.; Chen, X., (1998) “Improved Expression of Brevibacterium sterolicum Cholesterol Oxidase in Escherichia coli by Genetic Modification,” Prot. Exp. Purific., 12, 347-352. DOI:
Sampson, N. S.; McCann, A. E., (1997) “4,5-Cyclopropano-Cholestan-3b-Ol Substrates for Cholesterol Oxidase and Their 1H NMR Assignments, ” J. Org. Chem., 62, 5893 -5897. DOI:
Pyluck, A.; Ruiyong, Y.; Galligan Jr, E.; Primakoff, P.; Myles, D. G.; Sampson, N. S., (1997) “ECD Peptides Inhibit In Vitro Fertilization in Mice,” Bioorg. Med. Chem. Lett., 7, 1053-1058. DOI:
Ghoshroy, K. B.; Zhu, W.; Sampson, N. S., (1997) “Investigation of Membrane Disruption in the Reaction Catalyzed by Cholesterol Oxidase,” Biochemistry, 36, 6133-6140. DOI:
Sampson, N. S.; Kass, I. J., (1997) “Isomerization but not Oxidation is Suppressed by a Single Point Mutation, E361Q, in the Reaction Catalyzed by Cholesterol Oxidase,” J. Am. Chem. Soc., 119, 855-862. DOI:
Kass, I. J.; Sampson, N. S., (1995) “The Isomerization Catalyzed by Brevibacterium sterolicum Cholesterol Oxidase Proceeds Stereospecifically with One Base,” Biochem. Biophys. Res. Commun., 206, 688-693. DOI:
Sampson, N. S.; Knowles, J. R., (1992) “Segmental Motion in Catalysis: Investigation of a Critical Hydrogen Bond for Loop Closure in the Reaction of Triosephosphate Isomerase,” Biochemistry, 31, 8488-8494. DOI:
Sampson, N. S.; Knowles, J. R., (1992) “Segmental Movement: Definition of the Structural Requirements for Loop Closure in Catalysis by Triosephosphate Isomerase,” Biochemistry, 31, 8482-8487. DOI:
Sampson, N. S.; Bartlett, P. A., (1991) “Attempted de Novo Design, Synthesis, and Evaluation of a Ligand for the Allosteric Site of Phosphofructokinase,” J. Org. Chem. 56, 7179-7183. DOI:
Bone, R.; Sampson, N. S.; Bartlett, P. A.; Agard, D. A., (1991) “Crystal Structures of a-Lytic Protease Complexes with Irreversibly Bound Phosphonate Esters,” Biochemistry 30, 2263-2272. DOI:
Sampson, N. S.; Bartlett, P. A., (1991) “Peptidic Phosphonylating Agents as Irreversible Inhibitors of Serine Proteases and Models of the Tetrahedral Intermediates,” Biochemistry 30, 2255-2263. DOI:
Sampson, N. S.; Bartlett, P. A., (1988) “Synthesis of Phosphonic Acid Derivatives by Oxidative Activation of Phosphinate Esters,” J. Org. Chem. 53, 4500-4503. DOI:
Romoff, T. T.; Sampson, N. S.; van Eikeren, P., (1987) “Regioselectivity and Kinetics of Hydride Transfer in Substituted 1-Benzyl-3-quinolinecarboxamide Redox Reactions,” J. Org. Chem. 52, 4454-4459. DOI: